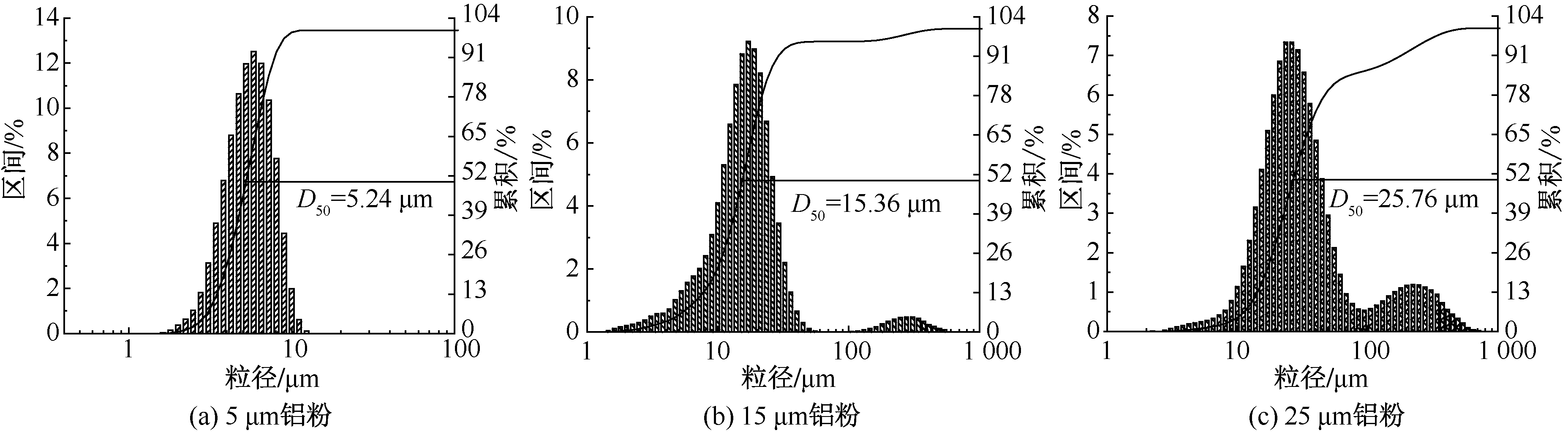
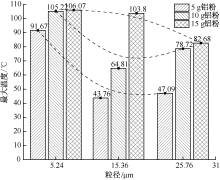
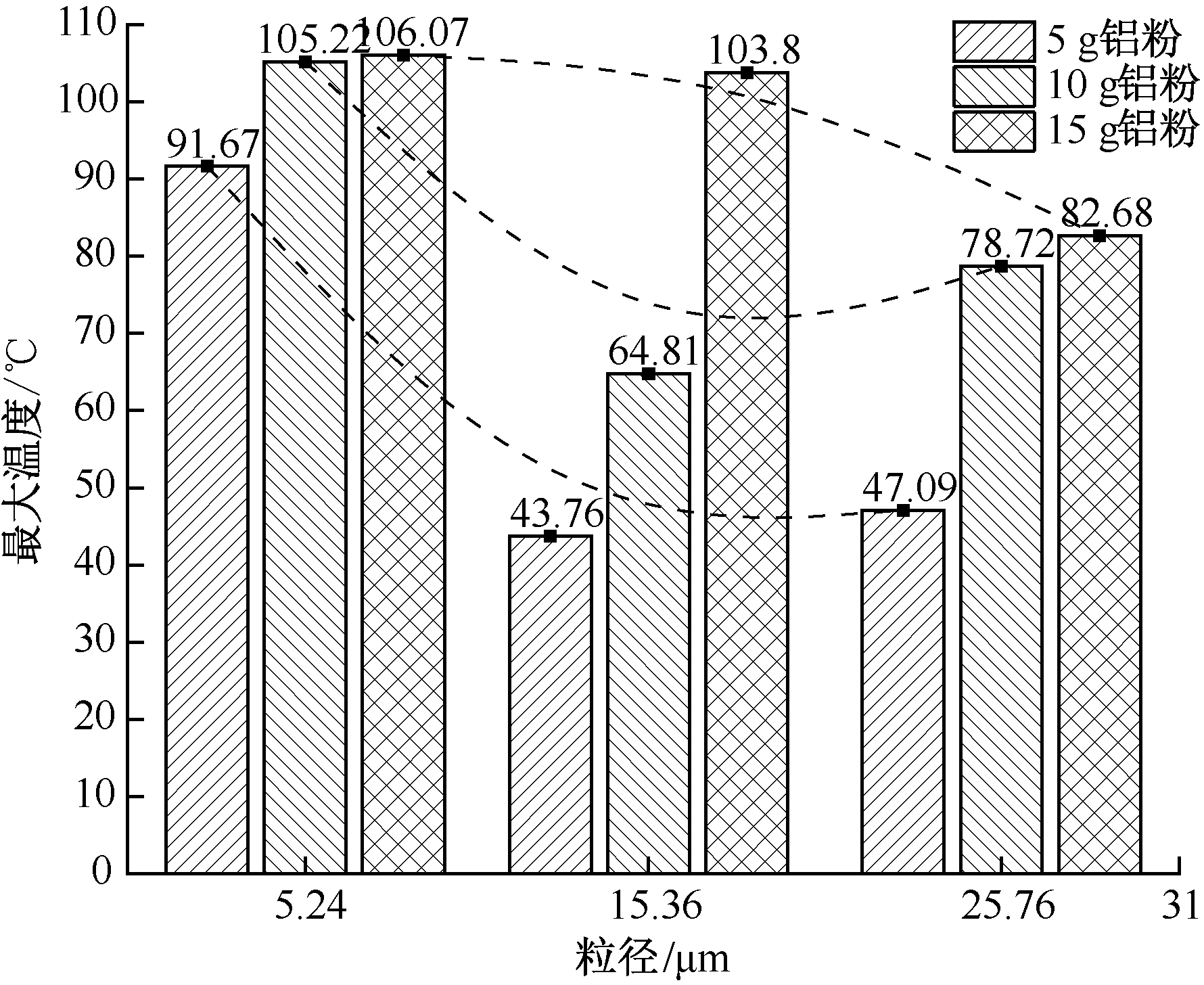
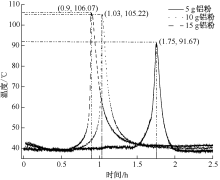
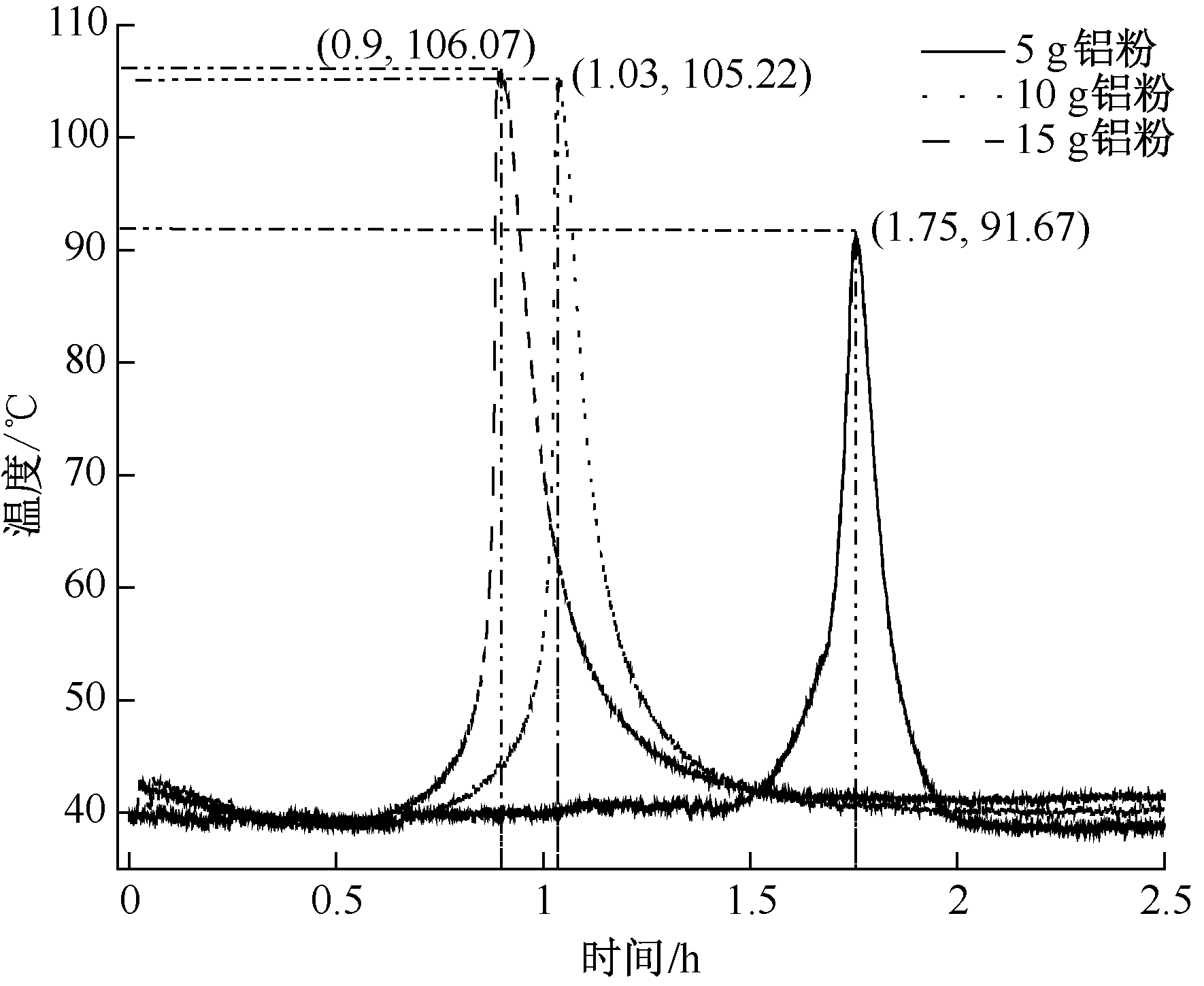
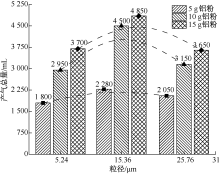
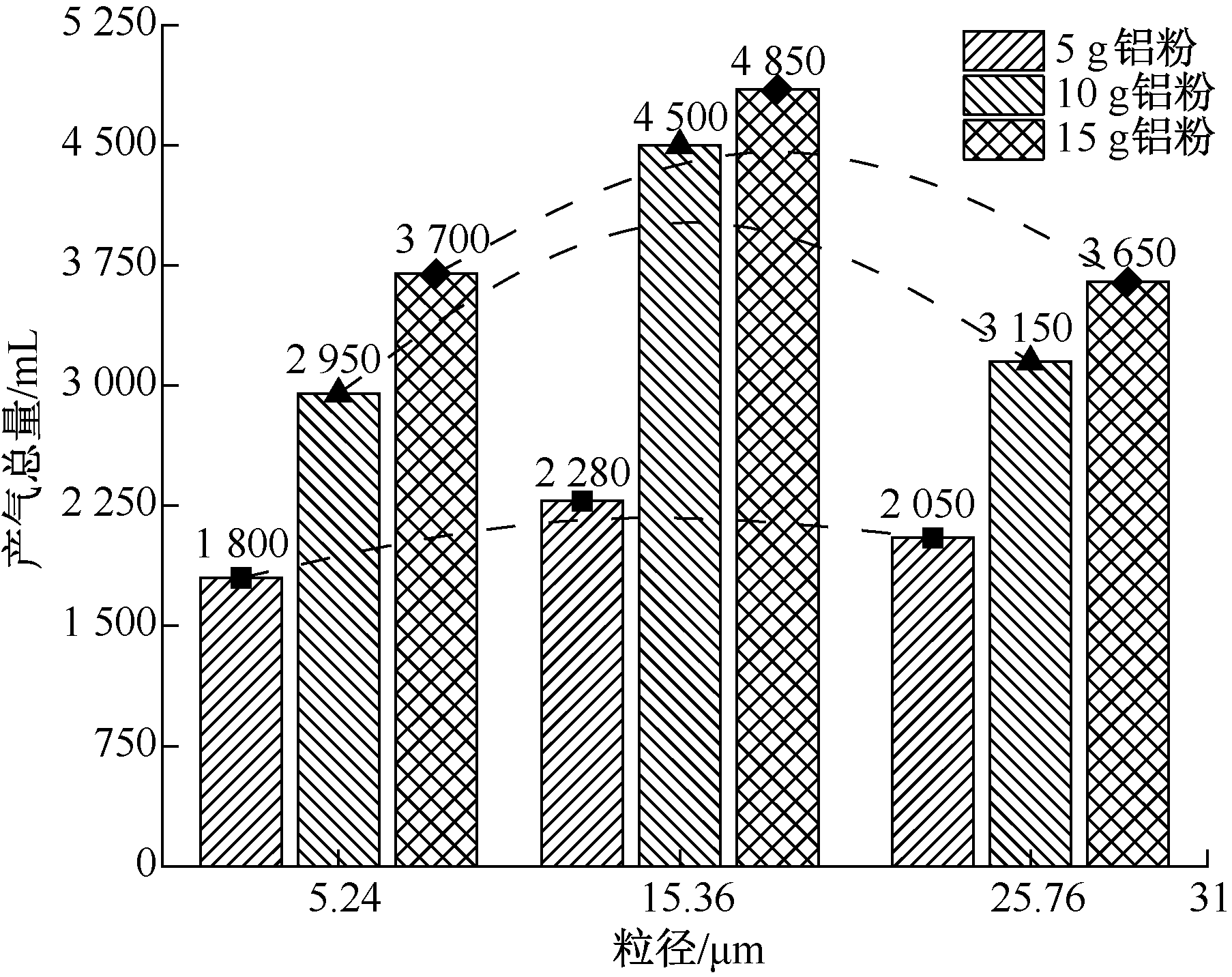
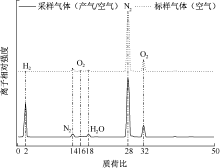
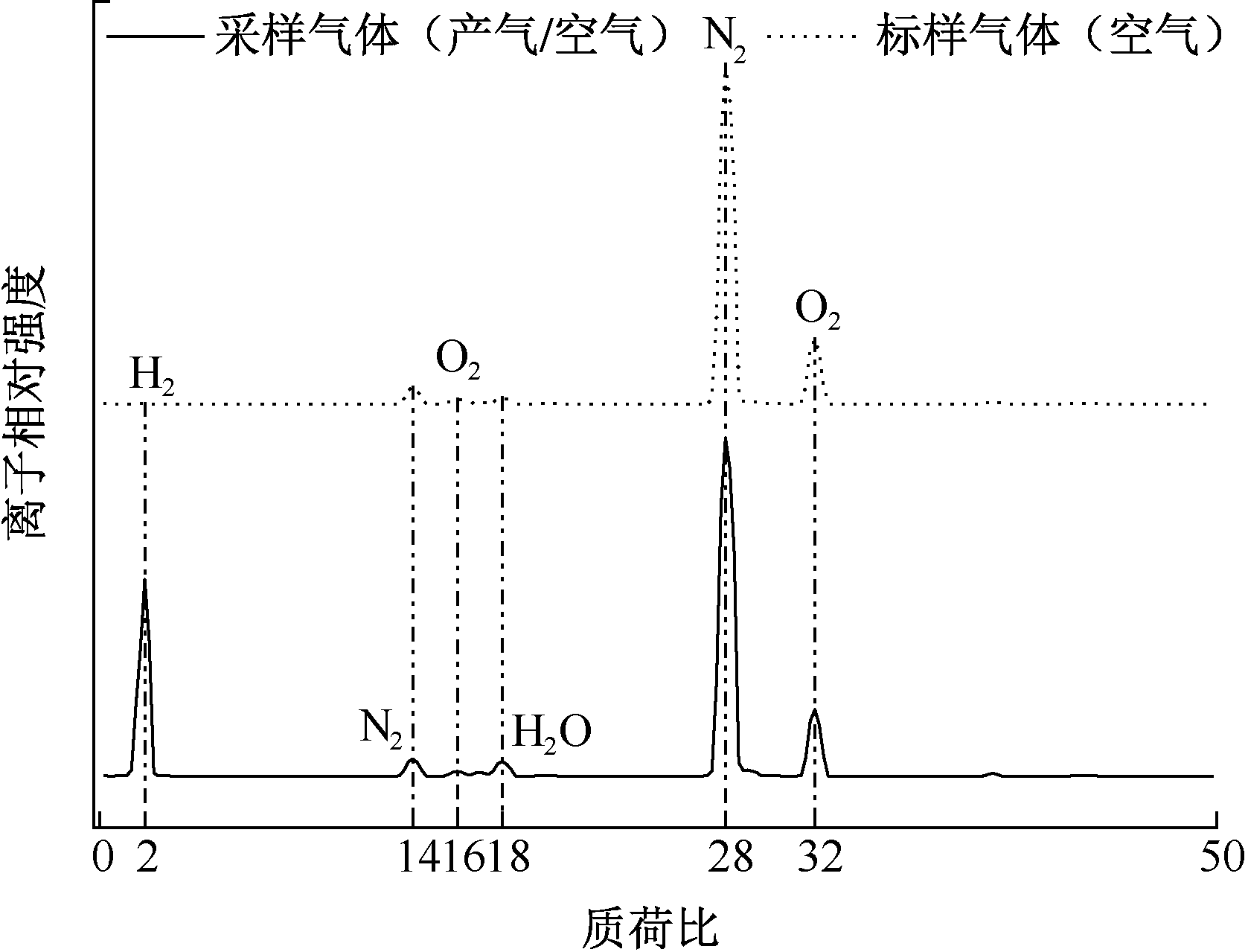
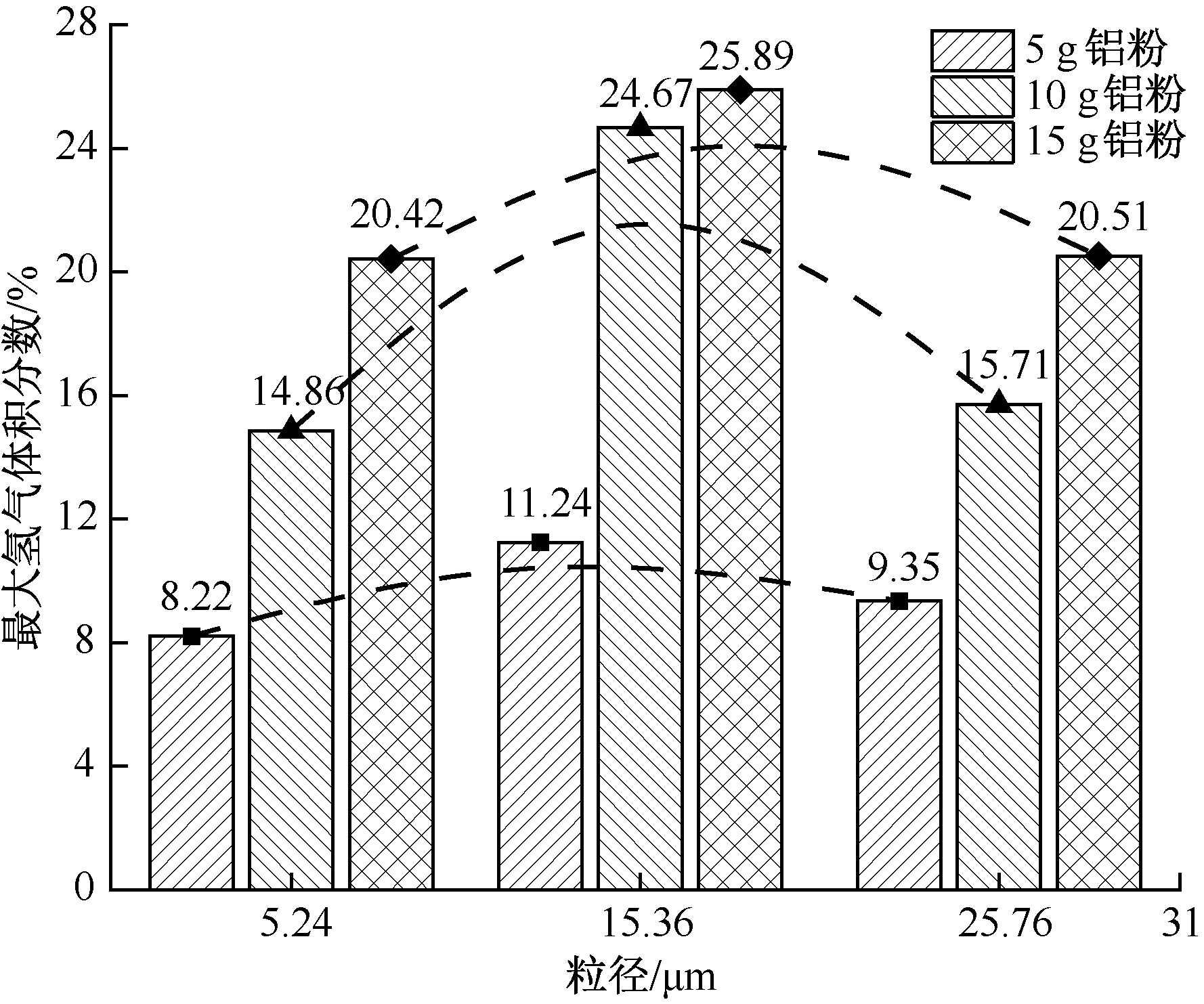
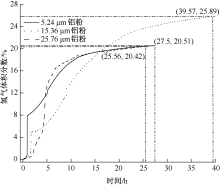
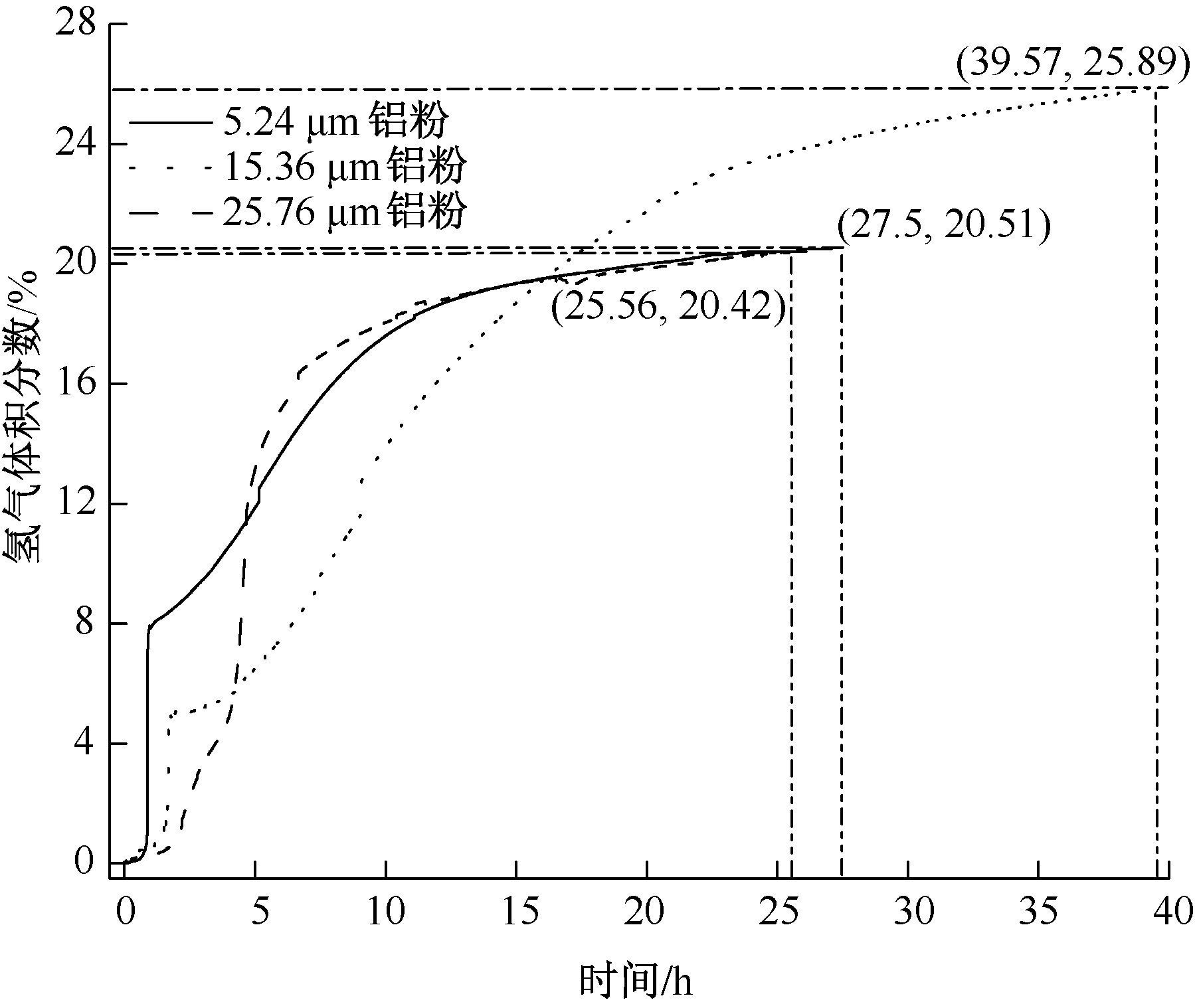
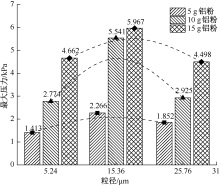
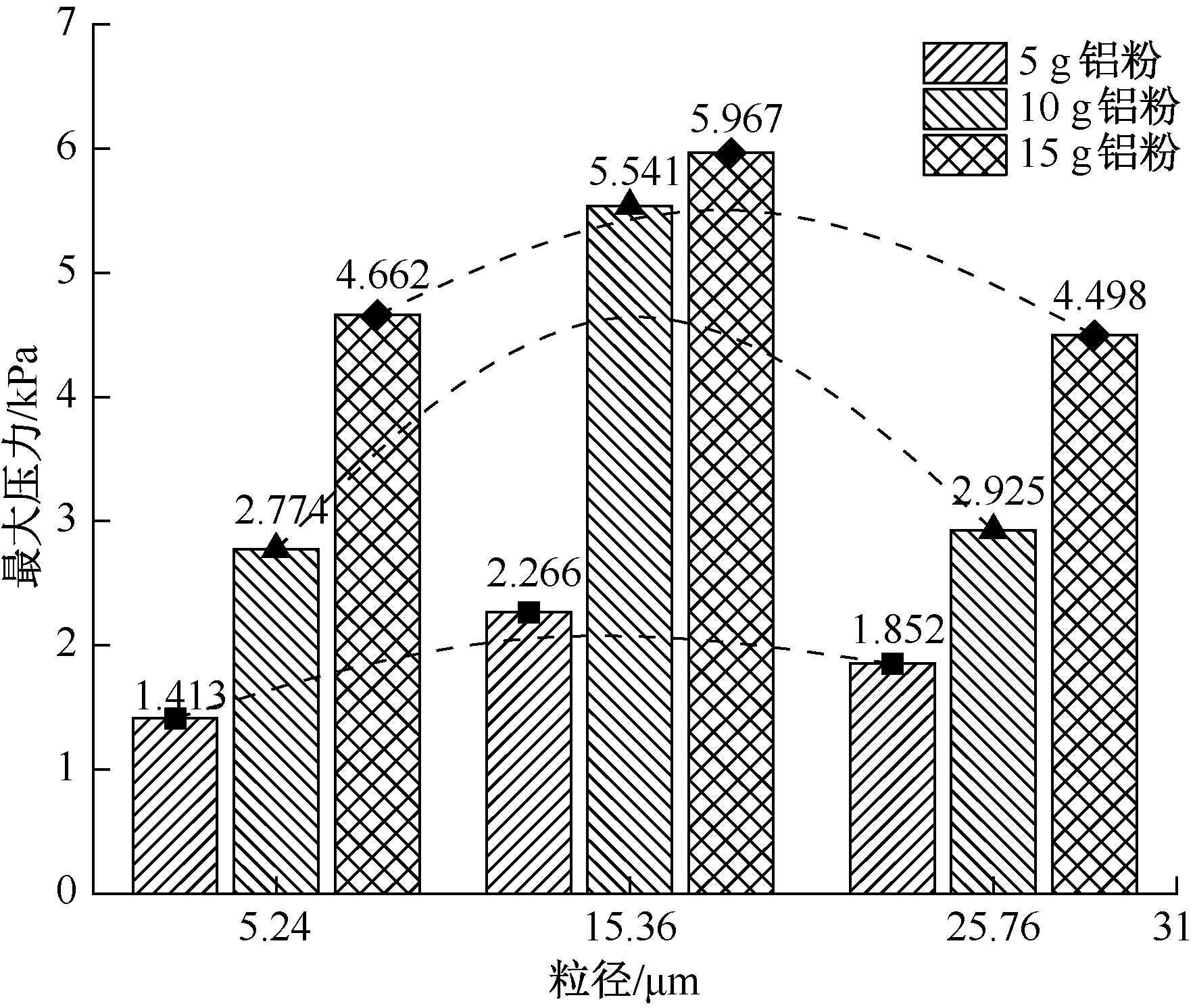
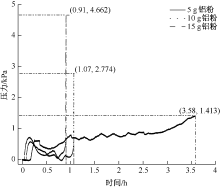
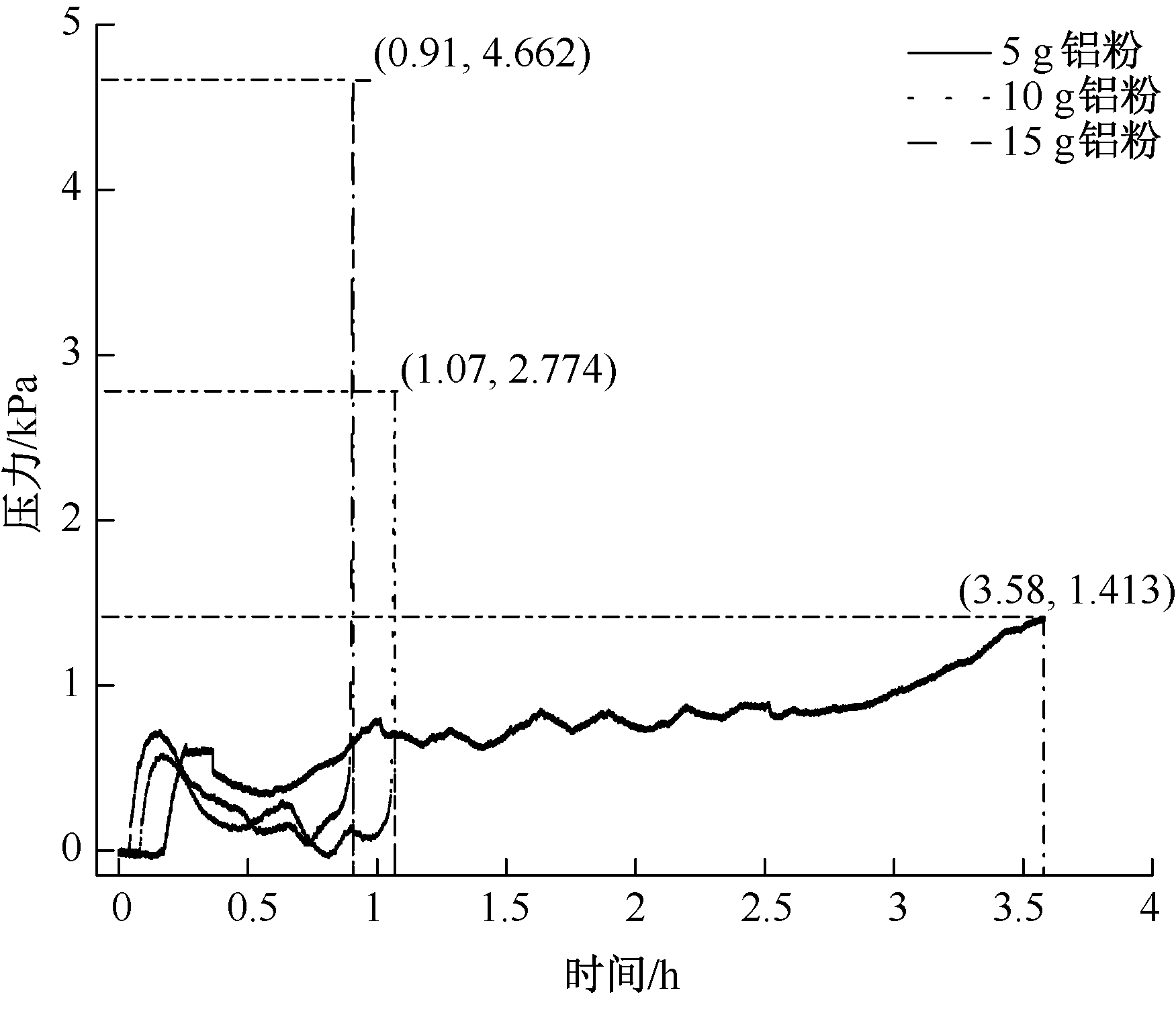
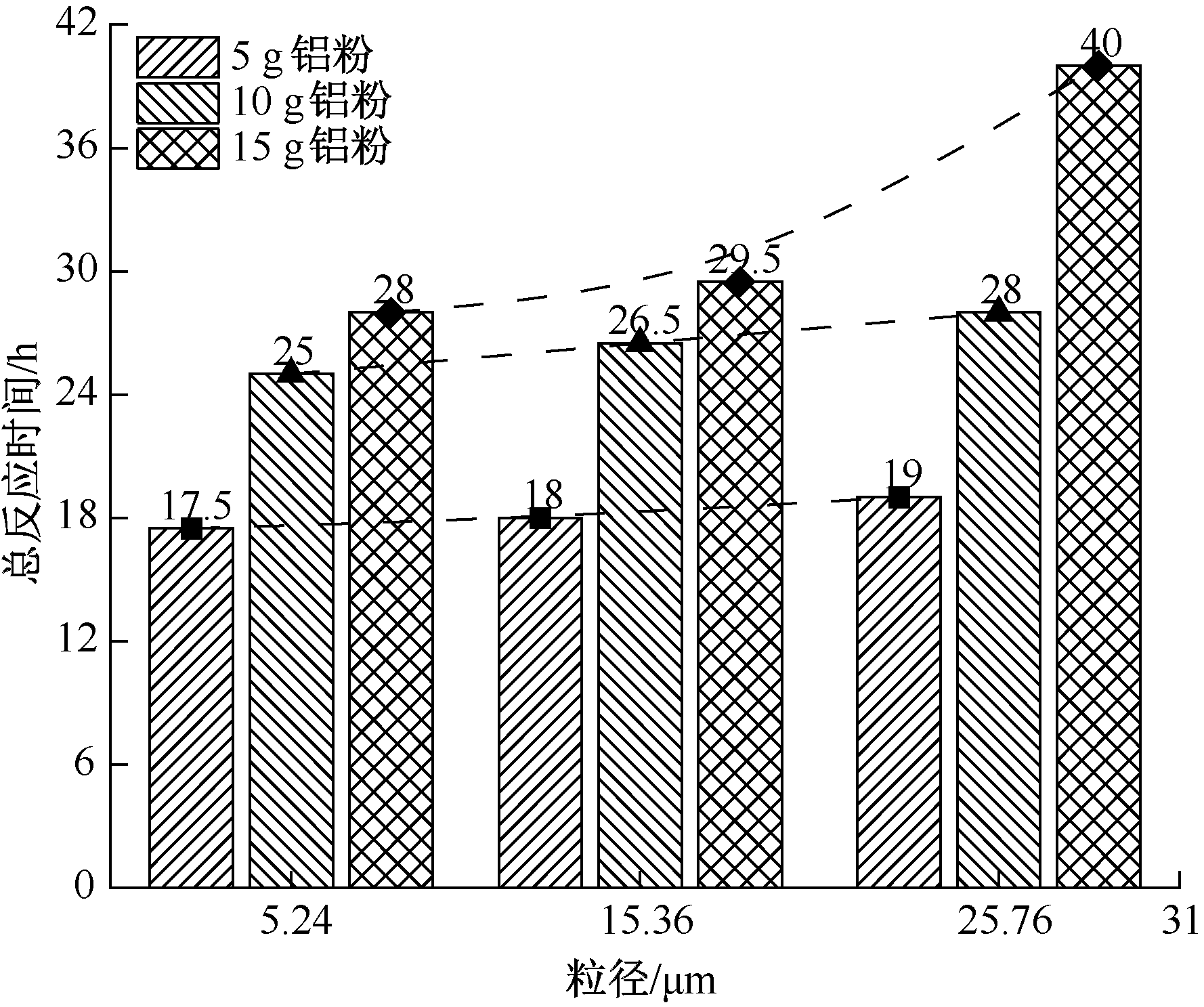
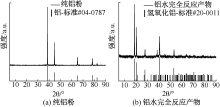
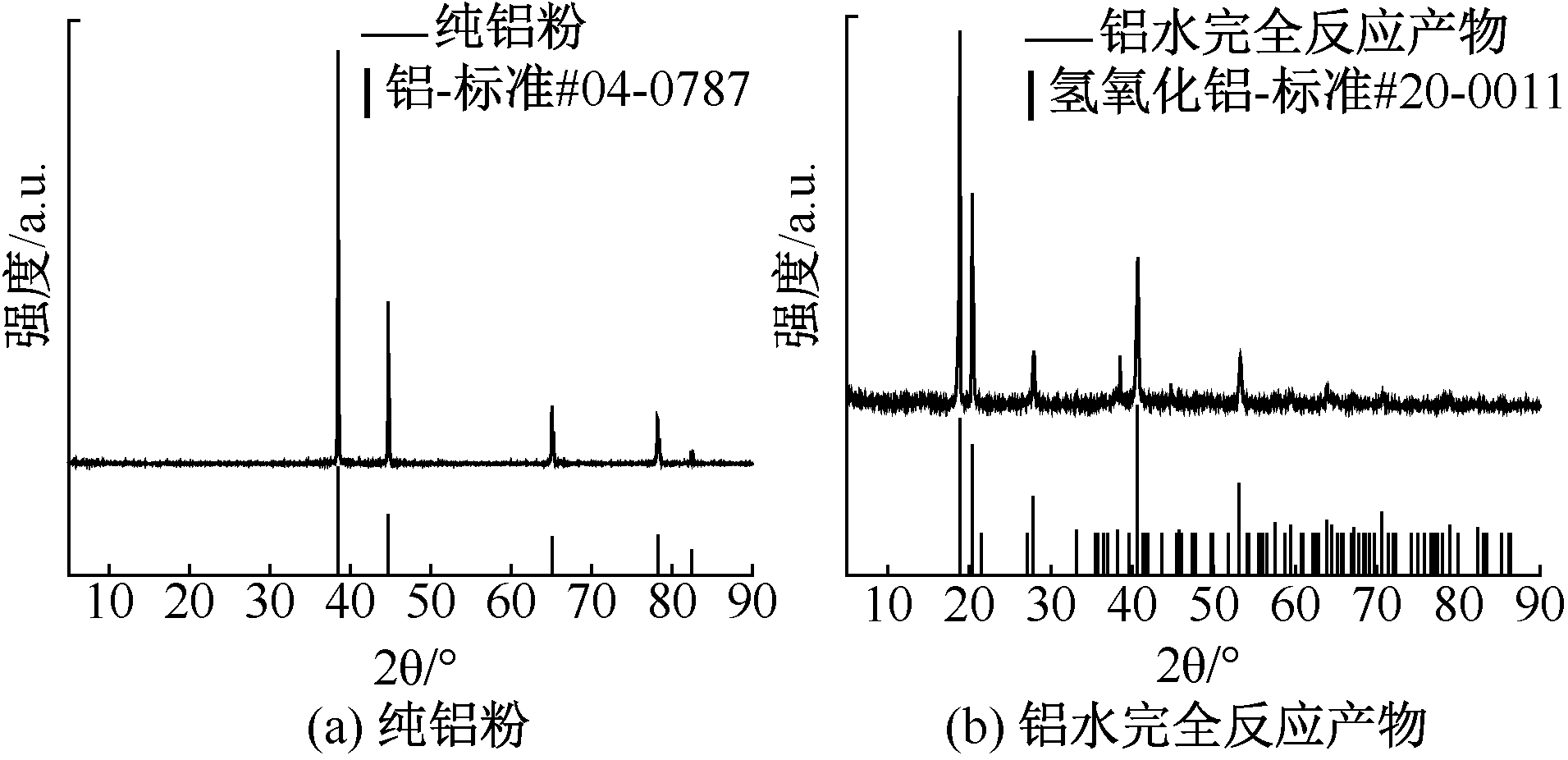
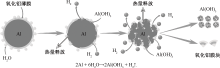
| [1] |
丁东梅. 铝粉遇水反应热动力学行为研究[D]. 上海: 上海应用技术大学, 2020.
|
|
DING Dongmei. Study on the thermodynamics of aluminum powder in water[D]. Shanghai: Shanghai Institute of Technology, 2020.
|
| [2] |
葛双优. 抛光铝粉热自燃机理研究[D]. 沈阳: 东北大学, 2015.
|
|
GE Shuangyou. Study on the mechanism of auto ignition of aluminum dust from polishing process[D]. Shenyang: Northeastern University, 2015.
|
| [3] |
刘聪聪, 许开立, 王雪, 等. 铝合金加工废物遇湿产氢规律研究[J]. 消防科学与技术, 2023, 42(4): 495-498.
|
|
LIU Congcong, XU Kaili, WANG Xue, et al. Study on hydrogen production law of aluminum alloy processing waste when wet[J]. Fire Science and Technology, 2023, 42(4): 495-498.
|
| [4] |
王鑫阳, 李刚. 水分促使抛光铝粉热自燃临界温度和诱导时间[J]. 东北大学学报:自然科学版, 2022, 43(10): 1492-1498.
|
|
WANG Xinyang, LI Gang. Critical temperature and induction time of spontaneous combustion of polished Al powder induced by water[J]. Journal of Northeastern University: Natural Science, 2022, 43(10): 1492-1498.
|
| [5] |
王鑫阳, 崔铁军, 王子健. 微米级铝粉产氢动力学试验及微观形貌变化规律研究[J]. 有色金属:中英文, 2025, 15(3): 368-375.
|
|
WANG Xinyang, CUI Tiejun, WANG Zijian. Experimental study on hydrogen production kinetics and microscopic morphology change of micron-sized aluminum powder[J]. Nonferrous Metals, 2025, 15(3): 368-375.
|
| [6] |
WANG Xinyang, LI Gang, ECKHOFF R K. Kinetics study of hydration reaction between aluminum powder and water based on an improved multi-stage shrinking core model[J]. International Journal of Hydrogen Energy, 2021, 46(67): 33 635-33 655.
|
| [7] |
NIE Hongqi, SCHOENITZ M, DREIZIN E L. Calorimetric investigation of the aluminum-water reaction[J]. International Journal of Hydrogen Energy, 2012, 37(15): 11 035-11 045.
|
| [8] |
庞磊, 李超, 钟圣俊, 等. 铝、镁及其合金粉遇湿反应动力学机理及产氢规律[J]. 北京理工大学学报, 2025, 45(11):1185-1193.
|
|
PANG Lei, LI Chao, ZHONG Shengjun, et al. Reaction kinetics mechanism and hydrogen production law of aluminum, magnesium andalloy powders upon exposure to moisture[J]. Transactions of Beijing Institute of Technology, 2025, 45(11): 1185-1193.
|
| [9] |
PANG Lei, LI Chao, LIU Jiqing, et al. Study on the hydrogen production law and reaction kinetics mechanism of aluminum powder oxidation under different moisture content conditions[J]. International Journal of Hydrogen Energy, 2025, 100: 319-329.
doi: 10.1016/j.ijhydene.2024.12.303
|
| [10] |
MAGUIRE J, WOODCOCK L V. Thermodynamics of tower-block infernos: effects of water on aluminum fires[J]. Entropy, 2019, 22(1): DOI: 10.3390/e22010014.
|
| [11] |
NISHIWAKI Y, SATO Y. Exothermic reaction of fine and coarse magnesium powders with water[J]. Science and Technology of Energetic Materials, 2022, 83(1): 20-27.
|
| [12] |
SACELEANU F, VUONG T V, MASTER E R, et al. Tunable kinetics of nanoaluminum and microaluminum powders reacting with water to produce hydrogen[J]. International Journal of Energy Research, 2019, 43(13): 7384-7396.
|
| [13] |
王延瞳, 许开立, 李力, 等. 铝颗粒与水反应产氢影响因素及抑制[J]. 东北大学学报:自然科学版, 2018, 39(5): 731-735.
|
|
WANG Yantong, XU Kaili, LI Li, et al. Influencing factors and inhibition of hydrogen produced by aluminum and water reaction[J]. Journal of Northeastern University: Natural Science, 2018, 39(5): 731-735.
|
| [14] |
邢婧, 毕跃, 崔瀚. 铝粉遇水燃烧爆炸危险性的实验研究[J]. 黑龙江科技大学学报, 2022, 32(2): 185-188.
|
|
XING Jing, BI Yue, CUI Han. Experimental study on risk of combustion and explosion of aluminum powder in water[J]. Journal of Heilongjiang University of Science and Technology, 2022, 32(2): 185-188.
|
| [15] |
盖卫卓. 纯铝粉体与水反应产氢及其相关影响因素的研究[D]. 上海: 上海大学, 2014.
|
|
GAI Weizhuo. Hydrogen-generation by the reaction of pure Al powder with water and the related influencing factors[D]. Shanghai: Shanghai University, 2014.
|