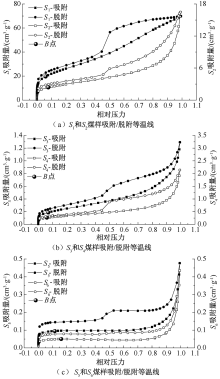
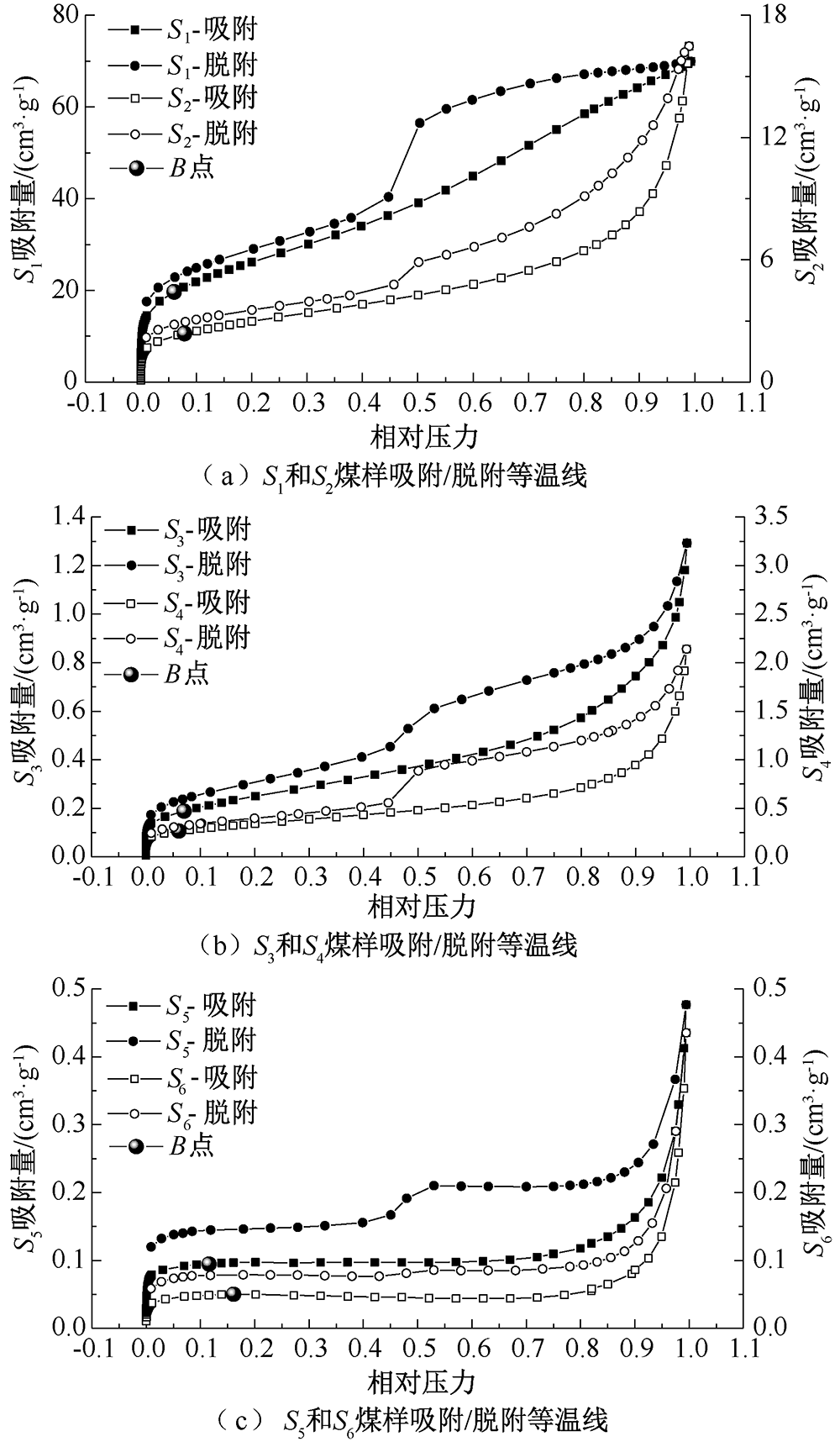
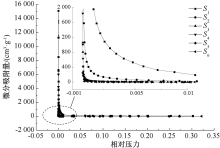
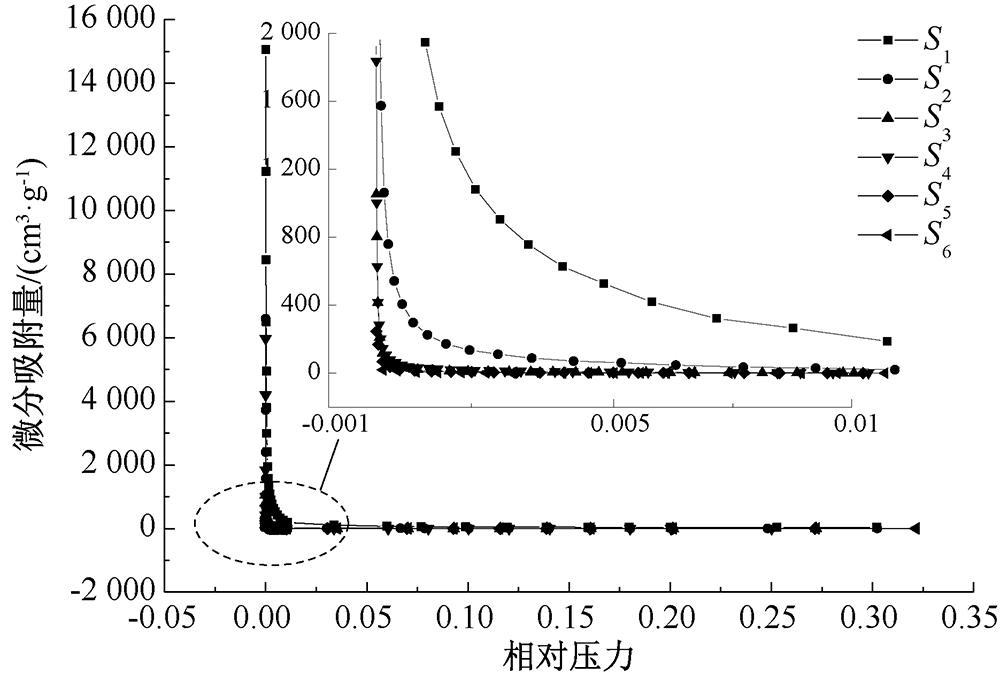
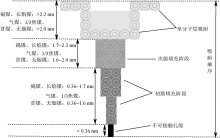
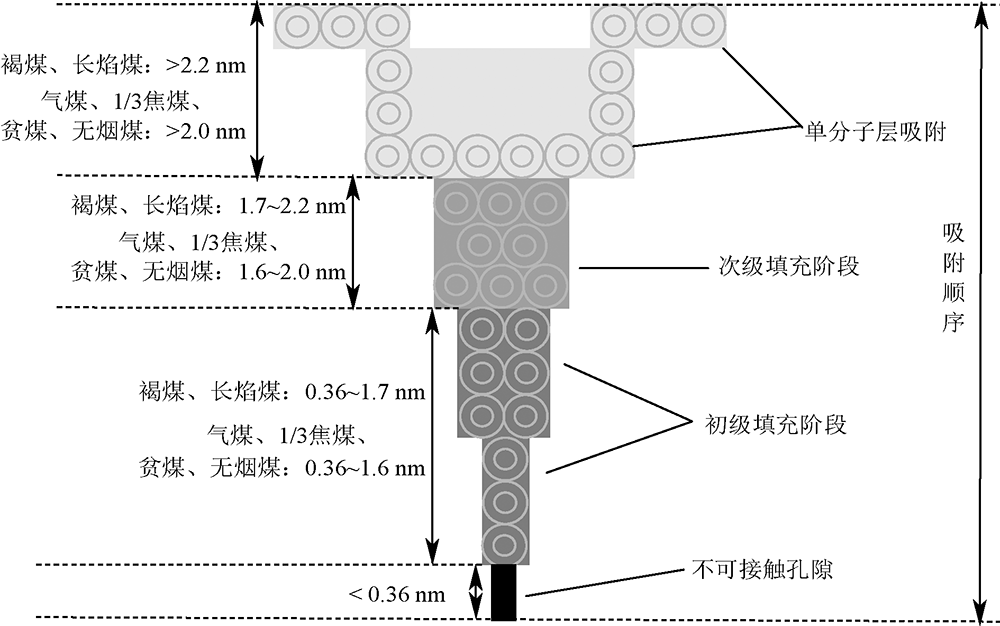
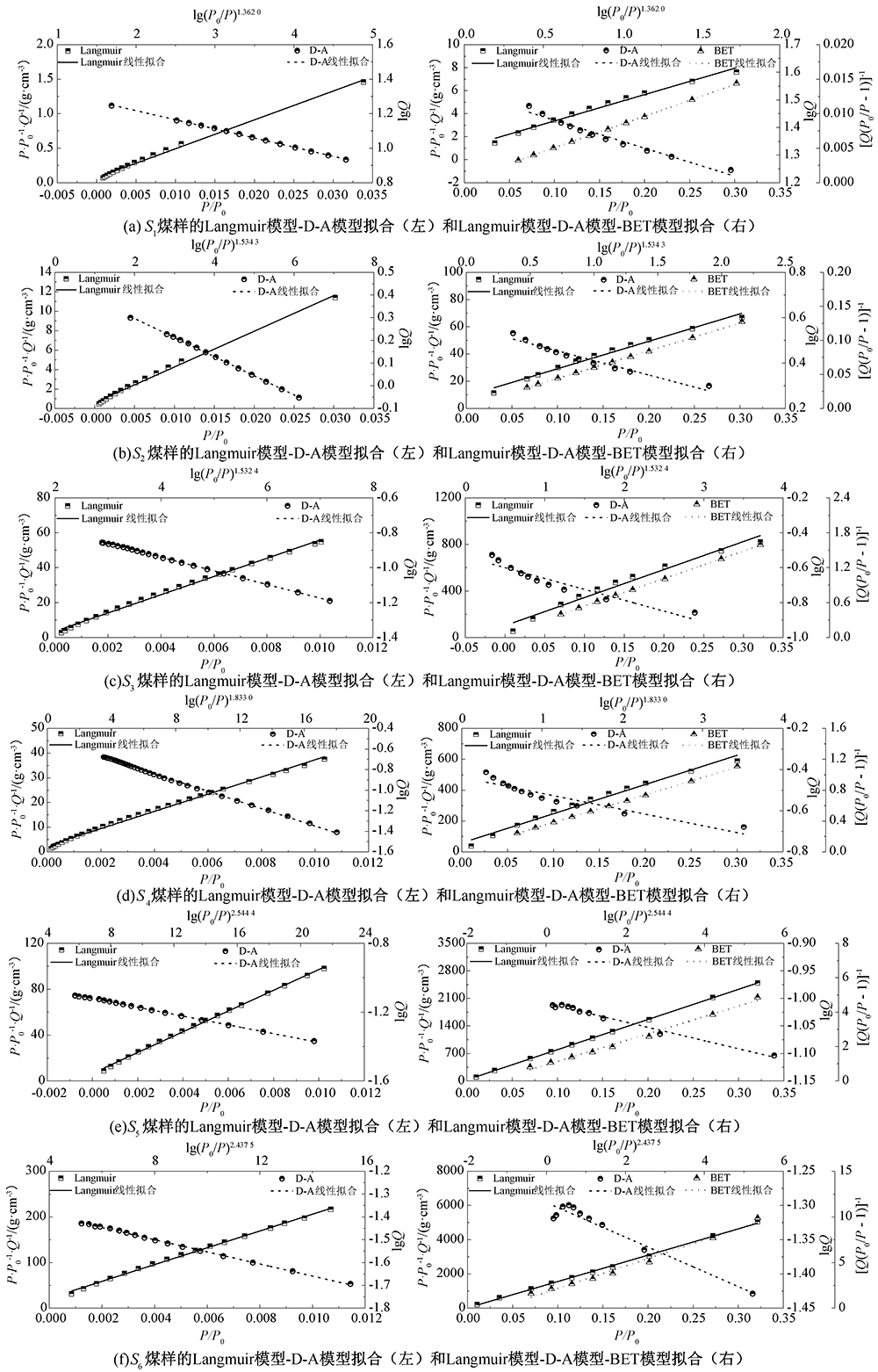
| [1] |
洪林. 碳材料孔隙吸附性能及突出煤孔隙特征研究[D]. 葫芦岛: 辽宁工程技术大学, 2014.
|
|
HONG Lin. Research on the adsorptivity of pore in caron materials and the porosity character of outbust coal[D]. Huludao: Liaoning Technical University, 2014.
|
| [2] |
刘佳佳, 胡建敏, 杨明, 等. 不同层理高阶煤孔隙特征的核磁共振试验[J]. 中国安全科学学报, 2021, 31(9):83-89.
doi: 10.16265/j.cnki.issn1003-3033.2021.09.012
|
|
LIU Jiajia, HU Jianmin, YANG Ming, et al. Nuclear magnetic resonance experimental study on pore characteristics of high rank coal with different bedding[J]. China Safety Science Journal, 2021, 31(9):83-89.
doi: 10.16265/j.cnki.issn1003-3033.2021.09.012
|
| [3] |
赵迪斐, 卢琪荣, 郭英海, 等. 煤层气储层孔隙纳米尺度表征的研究进展与展望:精细、量化与机理耦合[J]. 非常规油气, 2020, 7(1):108-118,100.
|
|
ZHAO Difei, LU Qirong, GUO Yinghai, et al. Research progress and prospects of pore nano scale characterization of coalbed methane reservoir: accurate characterization, quantification and mechanism coupling[J]. Unconventional Oil & Gas, 2020, 7(1):108-188,100.
|
| [4] |
李阳, 张玉贵, 张浪, 等. 基于压汞、低温N2吸附和CO2吸附的构造煤孔隙结构表征[J]. 煤炭学报, 2019, 44(4):1188-1196.
|
|
LI Yang, ZHANG Yugui, ZHANG Lang, et al. Characterization on pore structure of tectonic coals based on the method of mercury intrusion, carbon dioxide adsorption and nitrogen adsorption[J]. Journal of China Coal Society, 2019, 44(4):1188-1196.
|
| [5] |
THOMMES M, MORLAY C, AHMAD R, et al. Assessing surface chemistry and pore structure of active carbons by a combination of physisorption (H2O, Ar, N2, CO2), XPS and TPD-MS[J]. Adsorption, 2011, 17(3):653-661.
doi: 10.1007/s10450-011-9360-4
|
| [6] |
XIONG Qingrong, LI Kang, YANG Diansen, et al. Characterizing coal pore space by gas adsorption, mercury intrusion, FIB-SEM and μ-CT[J]. Environmental Earth Sciences, 2020, 79(10):209-224.
doi: 10.1007/s12665-020-08950-3
|
| [7] |
闫江伟, 薄增钦, 杨亚磊. 纳米级孔隙对构造煤吸附瓦斯能力的影响[J]. 中国安全科学学报, 2018, 28(10):131-136.
doi: 10.16265/j.cnki.issn1003-3033.2018.10.022
|
|
YAN Jiangwei, BO Zengqin, YANG Yalei. Influence of nanoscale pore on gas adsorption capacity of deformed coal[J]. China Safety Science Journal, 2018, 28(10):131-136.
doi: 10.16265/j.cnki.issn1003-3033.2018.10.022
|
| [8] |
洪林, 高大猛, 王继仁, 等. 低温低压下煤微孔吸附特性研究[J]. 中国安全科学学报, 2018, 28(12):77-82.
doi: 10.16265/j.cnki.issn1003-3033.2018.12.013
|
|
HONG Lin, GAO Dameng, WANG Jiren, et al. Adsorption characteristics of micropores in coal at low temperature and pressure[J]. China Safety Science Journal, 2018, 28(12):77-82.
doi: 10.16265/j.cnki.issn1003-3033.2018.12.013
|
| [9] |
近藤精一, 石川达雄, 安部郁夫. 吸附科学(原著第2版)[M].李国希,译. 北京: 化学工业出版社, 2006: 40-93.
|
| [10] |
GIL A. Analysis of the micropore structure of various microporous materials from nitrogen adsorption at 77 K[J]. Adsorption, 1998, 4(3/4):197-206.
doi: 10.1023/A:1008821430432
|
| [11] |
GHOSAL R, SMITH D M. Micropore characterization using the Dubinin-Astakhov equation to analyze high pressure CO2(273 K) adsorption data[J]. Journal of Porous Material, 1996, 3(4):247-255.
doi: 10.1007/BF01137914
|
| [12] |
WU Fengchin, WU Pinhsueh, TSENG Ruling, et al. Description of gas adsorption isotherms on activated carbons with heterogeneous micropores using the Dubinin-Astakhov equation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(4):1757-1763.
doi: 10.1016/j.jtice.2014.01.016
|
| [13] |
SHKOLIN A V, FOMKIN A A. Theory of volume filling of micropores applied to the description of methane adsorption on the microporous carbon adsorbent AUK[J]. Russian Chemical Bulletin, 2009, 58(4):717-721.
doi: 10.1007/s11172-009-0083-6
|
| [14] |
程远平, 胡彪. 微孔填充—煤中甲烷的主要赋存形式[J]. 煤炭学报, 2021, 46(9):2933-2948.
|
|
CHENG Yuanping, HU Biao. Main occurrence form of methane in coal: micropore filling[J]. Journal of China Coal Society, 2021, 46(9):2933-2948.
|
| [15] |
POLYAKOV N S, PETUKHOVA G A. Extension of the theory of volume filling of micropores to adsorption in supermicropores[J]. Adsorption, 2005, 11(3/4):357-362.
doi: 10.1007/s10450-005-5424-7
|
| [16] |
BALZER C, CIMINO R T, GOR G Y, et al. Deformation of microporous carbons during N2, Ar, and CO2 adsorption: insight from the density functional theory[J]. Langmuir, 2016, 32(32):8265-8274.
doi: 10.1021/acs.langmuir.6b02036
|
| [17] |
NAKAI K, NAKADA Y, HAKUMAN M, et al. High resolution N2 adsorption isotherms at 77.4 K and 87.3 K by carbon blacks and activated carbon fibers-analysis of porous texture of activated carbon fibers by alS1as-method[J]. Journal of Colloid and Interface Science, 2012, 367(1):383-393.
doi: 10.1016/j.jcis.2011.10.061
|
| [18] |
AN Fenghua, CHENG Yuanping, WU Dongmei, et al. The effect of small micropores on methane adsorption of coals from Northern China[J]. Adsorption, 2012, 19(1):83-90.
doi: 10.1007/s10450-012-9421-3
|
| [19] |
陈刘瑜, 李希建, 沈仲辉, 等. 贵州北部突出煤的孔隙结构及分形特征研究[J]. 中国安全科学学报, 2020, 30(2):66-72.
doi: 10.16265/j.cnki.issn1003-3033.2020.02.011
|
|
CHEN Liuyu, LI Xijian, SHEN Zhonghui, et al. Pore structure and fractal characteristics of outburst coal in northern Guizhou[J]. China Safety Science Journal, 2020, 30(2):66-72.
doi: 10.16265/j.cnki.issn1003-3033.2020.02.011
|
| [20] |
GB/T 474—2008, 煤样的制备方法[S].
|
|
GB/T 474-2008, Method for preparation of coal sample[S].
|
| [21] |
GB/T 212—2008, 煤的工业分析方法[S].
|
|
GB/T 212-2008, Proximate analysis of coal[S].
|
| [22] |
EMMETT P H, BRUNAUER S. Accumulation of alkali promoters on surfaces of iron synthetic ammonia catalysts[J]. Journal of the American Chemical Society, 2002, 59(2):310-315.
doi: 10.1021/ja01281a026
|
| [23] |
张哲泠, 杨正红. 微介孔材料物理吸附准确性分析的理论与实践[J]. 催化学报, 2013, 34(10):1797-1810.
|
|
ZHANG Zheling, YANG Zhenghong. Theoretical and practical discussion of measurement accuracy for physisorption with micro- and mesoporous materials[J]. Chinese Journal of Catalysis, 2013, 34(10):1797-1810.
doi: 10.1016/S1872-2067(12)60601-9
|