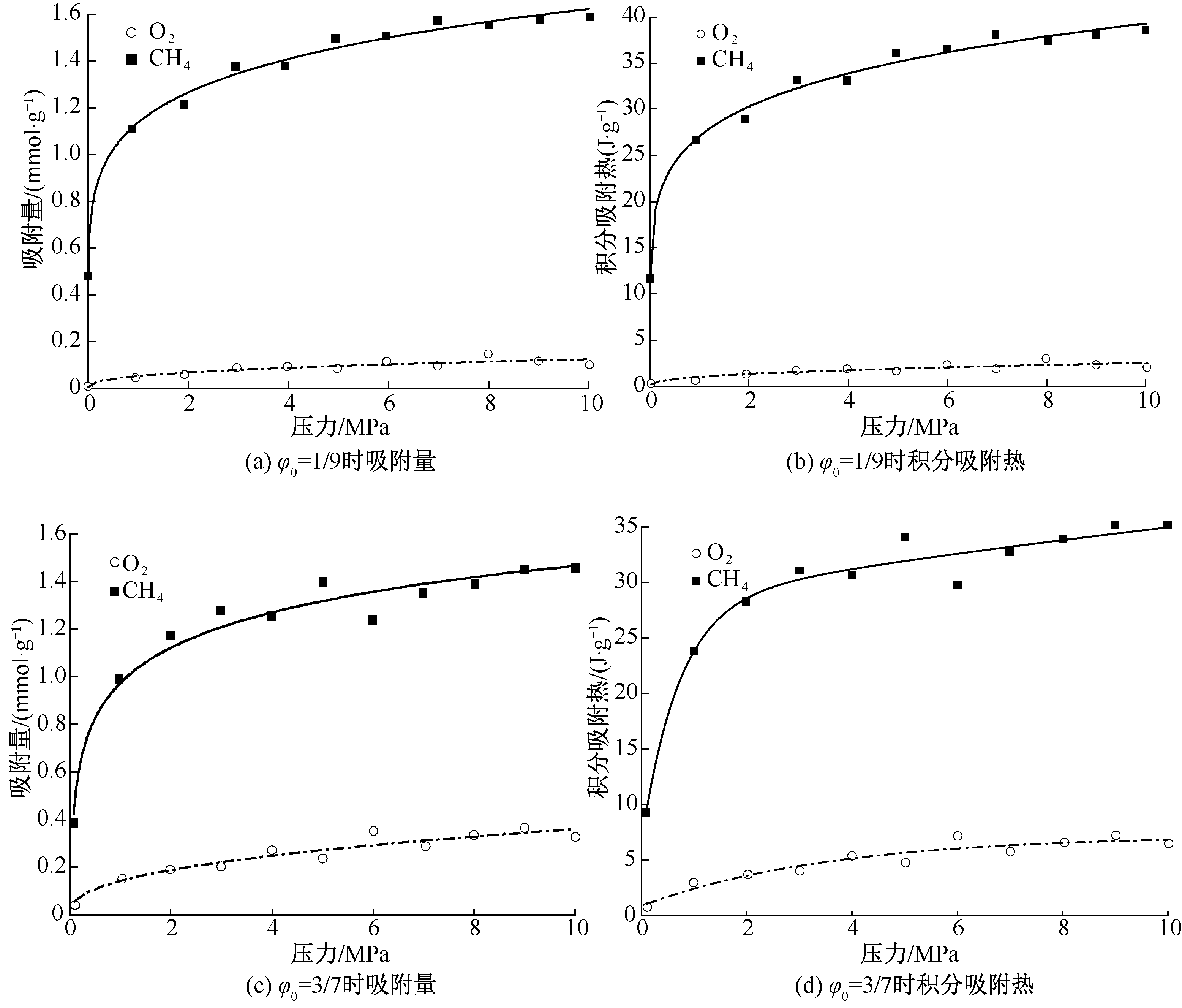
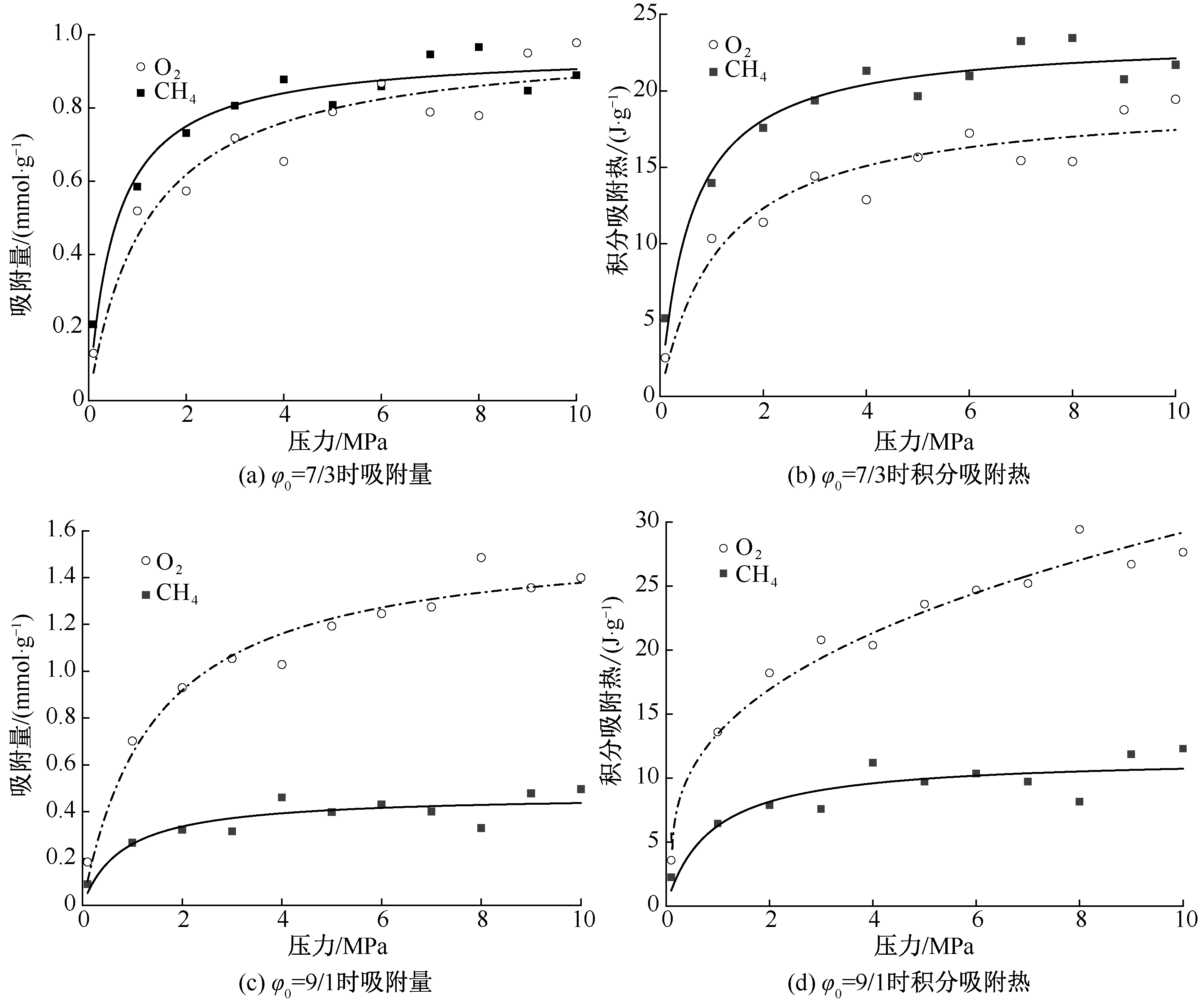
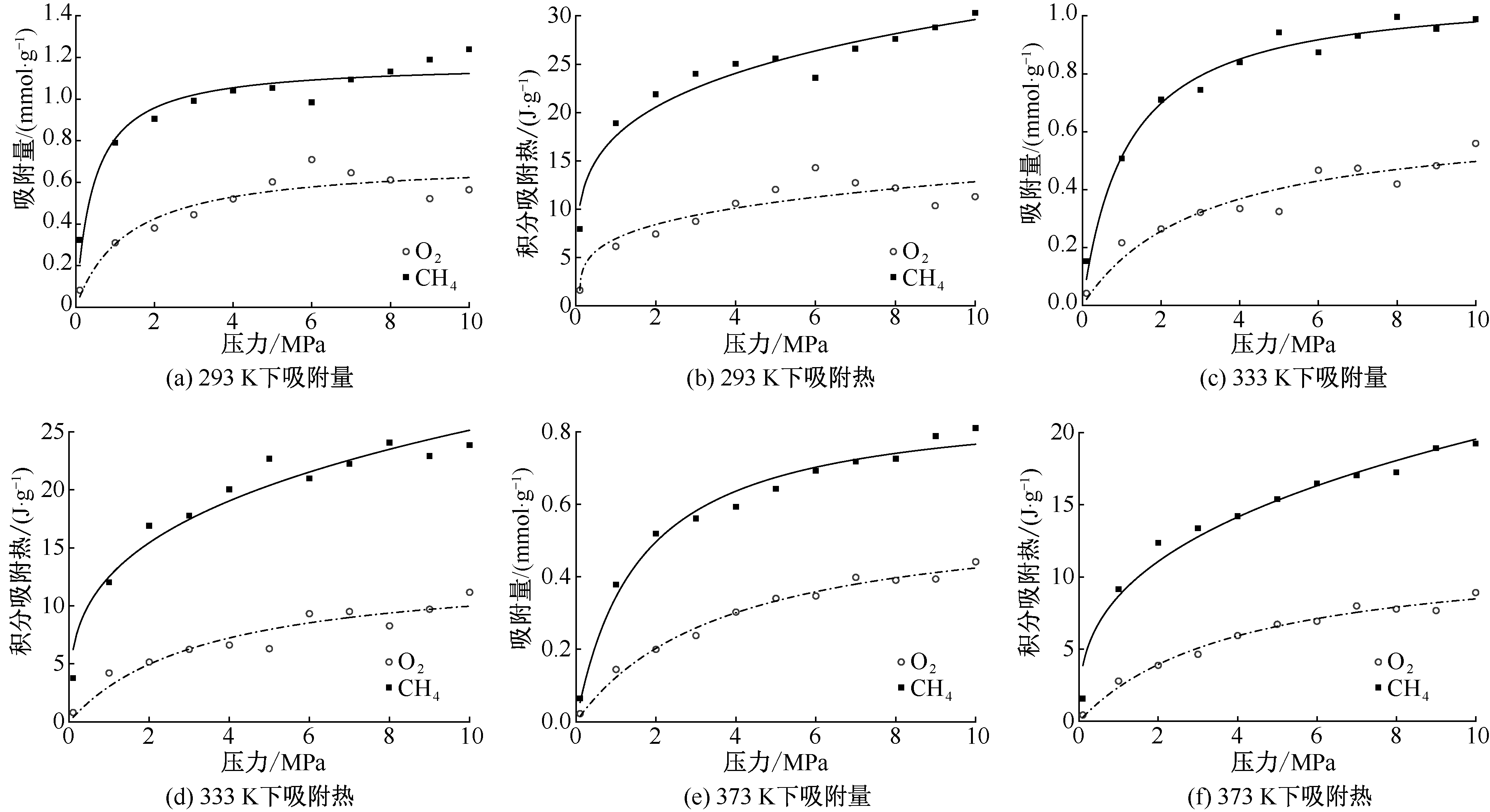
| [1] |
袁亮. 煤炭工业碳中和发展战略构想[J]. 中国工程科学, 2023, 25(5): 103-110.
doi: 10.15302/J-SSCAE-2023.05.009
|
|
YUAN Liang. Strategic Conception of carbon neutralization in coal industry[J]. China Academic Journal Electronic Publishing House, 2023, 25(5): 103-110.
|
| [2] |
程远平, 付建华, 俞启香. 中国煤矿瓦斯抽采技术的发展[J]. 采矿与安全工程学报, 2009, 26(2): 127-139.
|
|
CHENG Yuanping, FU Jianhua, YU Qixiang. Development of gas extraction technology in coal mines of China[J]. Journal of Mining &Safety Engineering, 2009, 26(2):127-139.
|
| [3] |
李沛, 马东民, 张辉, 等. 高、低阶煤润湿性对甲烷吸附/解吸的影响[J]. 煤田地质与勘探, 2016, 44(5): 80-85.
|
|
LI Pei, MA Dongmin, ZHANG Hui, et al. Influence of high and low rank coal wettability and methane adsorption / desorption characteristics[J]. Coal Geology & Exploration, 2016, 44(5):80-85.
|
| [4] |
田富超, 贾东旭, 陈明义, 等. 采空区复合灾害环境下含瓦斯煤自燃特征研究进展[J]. 煤炭学报, 2024, 49(6): 2711-2727.
|
|
TIAN Fuchao, JIA Dongxu, CHEN Mingyi, et al. Research progress of spontaneous combustion of coal containing gas under the compound disaster environment in the goaf[J]. Journal of China Coal Society, 2024, 49(6): 2711-2727.
|
| [5] |
ASKALANY A A, SAHA B B. Derivation of isosteric heat of adsorption for non-ideal gases[J]. International Journal of Heat and Mass Transfer, 2015, 89: 186-192.
|
| [6] |
XU Tang, RIPEPI N, STADIE N P, et al. Thermodynamic analysis of high pressure methane adsorption in Longmaxi shale[J]. Fuel, 2017, 193: 411-418.
|
| [7] |
刘志祥, 冯增朝. 煤体对瓦斯吸附热的理论研究[J]. 煤炭学报, 2012, 37(4):647-653.
|
|
LIU Zhixiang, FENG Zengchao. Theoretical study on adsorption heat of methane in coal[J]. Journal of China Coal Society, 2012, 37(4): 647-653.
|
| [8] |
KLOUTSE A F, ZACHARIAL R, COSSEMENT D, et al. Isosteric heat of hydrogen adsorption on MOFs: comparison between adsorption calorimetry, sorption isosteric method, and analytical models[J]. Applied Physics A, 2015, 121(4): 1417-1424.
|
| [9] |
申晓静, 岳基伟, 梁跃辉, 等. 高温高压氛围下煤体吸附瓦斯特性研究[J]. 中国安全科学学报, 2024, 34(2):176-184.
doi: 10.16265/j.cnki.issn1003-3033.2024.02.1453
|
|
SHEN Xiaojing, YUE Jiwei, LIANG Yuehui, et al. Study on gas adsorption characteristics of coal under high temperature and high pressure atmosphere[J]. China Safety Science Journal, 2024, 34(2):176-184.
doi: 10.16265/j.cnki.issn1003-3033.2024.02.1453
|
| [10] |
娄和壮, 贾廷贵. 惰性气氛对煤自燃过程的竞争吸附差异性研究[J]. 中国安全科学学报, 2020, 30(4):60-67.
doi: 10.16265/j.cnki.issn1003-3033.2020.04.010
|
|
LOU Hezhuang, JIA Tinggui. Competitive adsorption differences during coal spontaneous combustion process in noble gas Atmosphere[J]. China Safety Science Journal, 2020, 30(4):60-67.
doi: 10.16265/j.cnki.issn1003-3033.2020.04.010
|
| [11] |
仇悦, 龙航, 白杨, 等. 温度效应下煤体吸附瓦斯热力学及动力学特征[J]. 中国安全科学学报, 2023, 33(7):147-155.
doi: 10.16265/j.cnki.issn1003-3033.2023.07.1934
|
|
QIU Yue, LONG Hang, BAI Yang, et al. Thermodynamicand kinetic characteristics of gas adsorption by coal under temperature effect[J]. China Safety Science Journal, 2023, 33(7):147-155.
doi: 10.16265/j.cnki.issn1003-3033.2023.07.1934
|
| [12] |
DANG Yong, ZHAO Lianming, LU Xiaoqing, et al. Molecular simulation of CO2/CH4 adsorption in brown coal: effect of oxygen-, nitrogen-, and sulfur-containing functional groups[J]. Applied Surface Science, 2017, 423: 33-42.
|
| [13] |
王宝俊, 凌丽霞, 赵清艳, 等. 气体与煤表面吸附作用的量子化学研究[J]. 化工学报, 2009, 60(4): 196-201.
|
|
WANG Baojun, LING Lixia, ZHAO Qingyan, et al. Quantum chemistry study on adsorption of gases on coal surface[J]. CIESC Journal, 2009, 60(4): 196-201.
|
| [14] |
YOU Jing, TIAN Li, ZHANG Chao, et al. Adsorption behavior of carbon dioxide and methane in bituminous coal: a molecular simulation study[J]. Chinese Journal of Chemical Engineering, 2016, 24(9): 1275-1282.
|
| [15] |
林柏泉, 何学秋, 王佳新. 气体吸附性与煤和瓦斯突出的机理[J]. 江苏煤炭, 1990(2): 11-15.
|
|
LIN Baiquan, HE Xueqiu, WANG Jiaxin. Gas adsorption and the mechanism of coal and gas protrusion[J]. Jiangsu Coal, 1990 (2): 11-15.
|
| [16] |
JIA Chengxia, YOU Chun, PAN Gang. Effect of temperature on the sorption and desorption of perfluorooctane sulfonate on humic acid[J]. Journal of Environmental Sciences, 2010, 22(3): 355-361.
pmid: 20614776
|
| [17] |
杨雪莹, 朱一民, 李艳军, 等. 捕收剂DXY-1在石英表面的捕收性能及机理研究[J]. 金属矿山, 2020(6): 110-113.
|
|
YANG Xueying, ZHU Yimin, LI Yanjun, et al. Collecting performance and mechanism of collector DXY-1 on quartz surface[J]. Metal Mine, 2020(6): 110-113.
|
| [18] |
张景奇, 张覃, 卯松. 油酸根离子在氟磷灰石和白云石表面吸附动力学与吸附热力学研究[J]. 矿冶工程, 2024, 44(1): 60-67.
doi: 10.3969/j.issn.0253-6099.2024.01.014
|
|
ZHANG Jingqi, ZHANG Qin, MAO Song. Study on adsorption kinetics and adsorption thermodynamics of oleic acid ions on the surface of fluorapatite and dolomite[J]. Mining and Metallurgical Engineering, 2024, 44(1): 60-67.
doi: 10.3969/j.issn.0253-6099.2024.01.014
|
| [19] |
任鸣. 无定形纳米多孔材料的气体吸附仿真研究[D]. 北京: 北京化工大学, 2021.
|
|
REN Ming. Study on the adsorption of amorphous porous nanomaterials[D]. Beijing: Beijing University of Chemical Technology, 2021.
|
| [20] |
王学斌, 周澳, 杨明辉, 等. 燃煤电厂200 t/d生活垃圾无氧热解耦合协同处置优化[J]. 煤炭学报, 2022, 47(11): 3897-3905.
|
|
WANG Xuebin, ZHOU Ao, YANG Minghui, et al. Optimization on 200 t/ d garbage co-utilisation in a coal-fired power plant through air-free pyrolysis process[J]. Journal of China Coal Society, 2022, 47(11): 3897-3905.
|