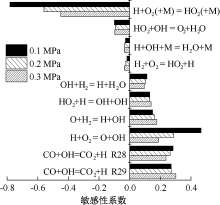
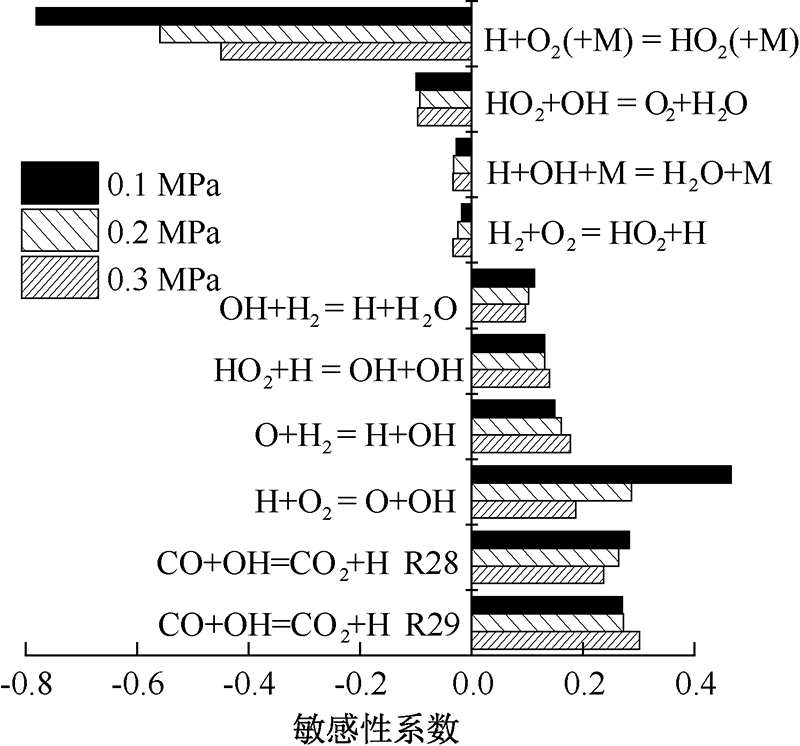
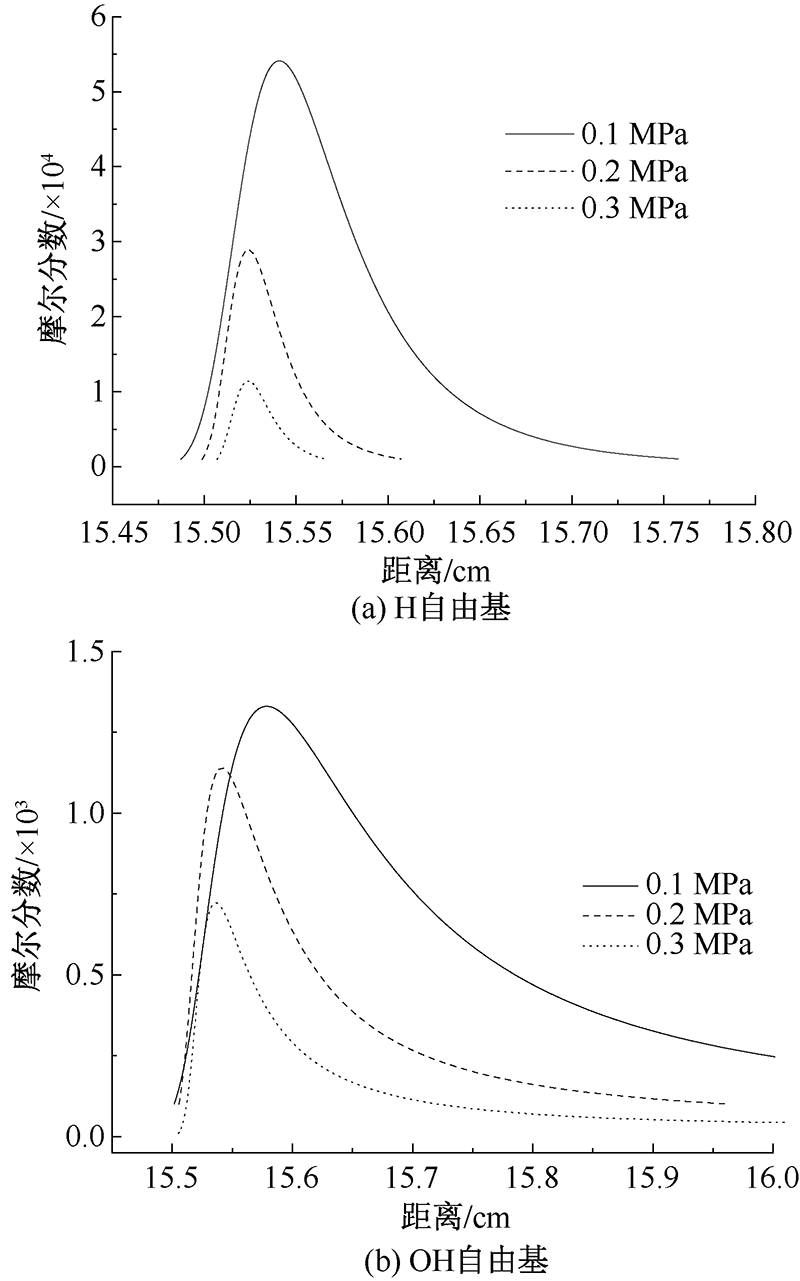
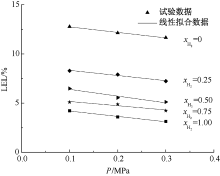
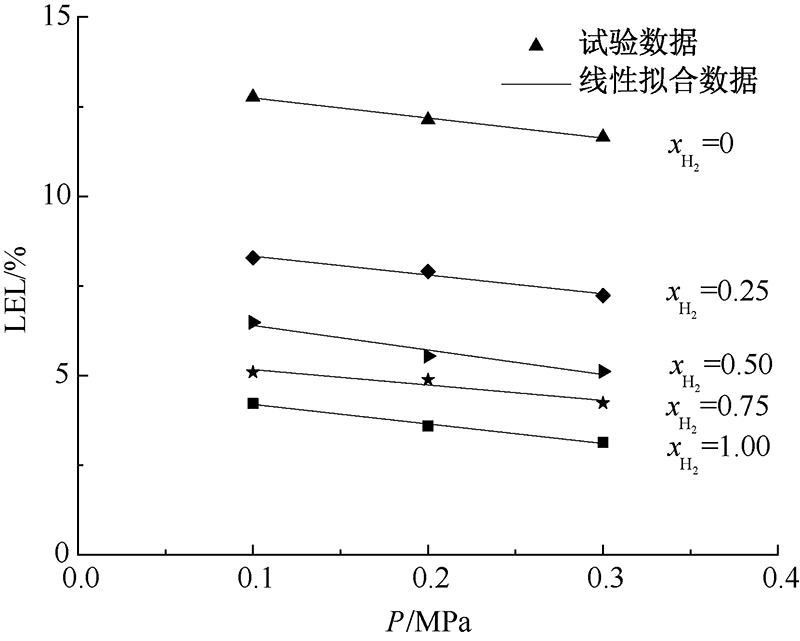
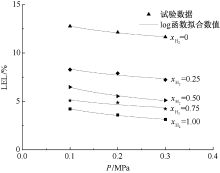
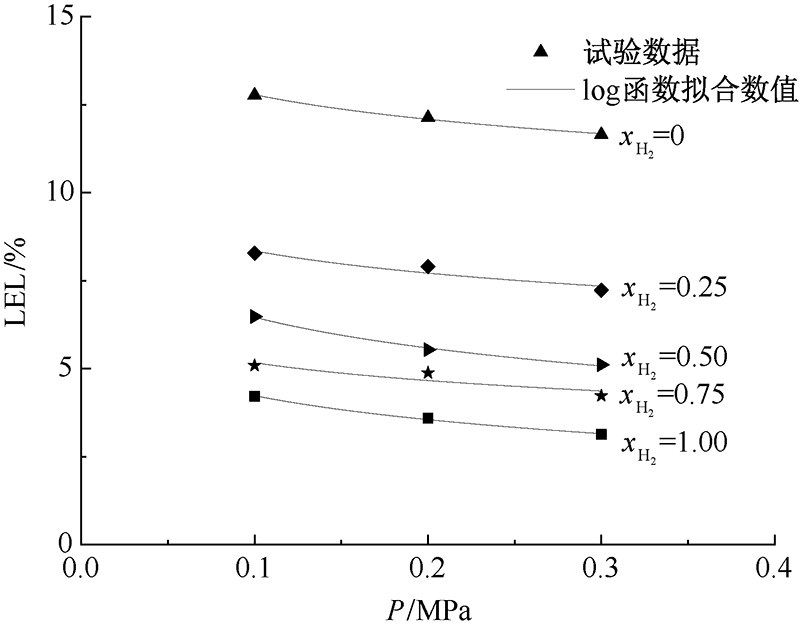
| [1] |
JAIMES D, MCDONELL V G, SAMUELSEN G S. Lean flammability limits of syngas/air mixtures at elevated temperatures and pressures[J]. Energy & Fuels, 2018, 32(10):10964-10973.
doi: 10.1021/acs.energyfuels.8b02031
|
| [2] |
E N1839: 2017, Determination of explosion limits of gases and vapours[S].
|
| [3] |
SCHOOR F, VERPLAETSEN F. The upper flammability limit of methane/hydrogen/air mixtures at elevated pressures and temperatures[J]. International Journal of Hydrogen Energy, 2007, 32(13):2548-2552.
doi: 10.1016/j.ijhydene.2006.10.053
|
| [4] |
WAN Xin, ZHANG Qi, SHEN Shilei.Theoretical estimation of the lower flammability limit of fuel-air mixtures at elevated temperatures and pressures[J]. Journal of Loss Prevention in the Process Industries, 2015, 36:13-19.
doi: 10.1016/j.jlp.2015.05.001
|
| [5] |
LI Pengliang, LIU Zhenyi, LI Mingzhi, et al. Experimental study on the flammability limits of natural gas/air mixtures at elevated pressures and temperatures[J]. Fuel, 2019, 256:DOI: 10.1016/j.fuel.2019.115950.
doi: 10.1016/j.fuel.2019.115950
|
| [6] |
CUI Gan, YANG Chao, LI Zili, et al. Experimental study and theoretical calculation of flammability limits of methane/air mixture at elevated temperatures and pressures[J]. Journal of Loss Prevention in the Process Industries, 2016, 41:252-258.
doi: 10.1016/j.jlp.2016.02.016
|
| [7] |
HUANG Lijuan, WANG Yu, PEI Shufeng, et al. Effect of elevated pressure on the explosion and flammability limits of methane-air mixtures[J]. Energy, 2019, 186:DOI: 10.1016/j.energy.2019.07.170.
doi: 10.1016/j.energy.2019.07.170
|
| [8] |
SARLI V D, BENEDETTO A D. Laminar burning velocity of hydrogen-methane/air premixed flames[J]. International Journal of Hydrogen Energy, 2007, 32(5):637-646.
doi: 10.1016/j.ijhydene.2006.05.016
|
| [9] |
DAVIS S G, JOSHI A V, WANG H, et al. An optimized kinetic model of H2/CO combustion[J]. Proceedings of the Combustion Institute, 2005, 30(1):1283-1292.
doi: 10.1016/j.proci.2004.08.252
|
| [10] |
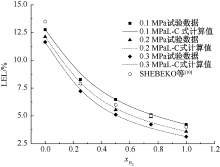
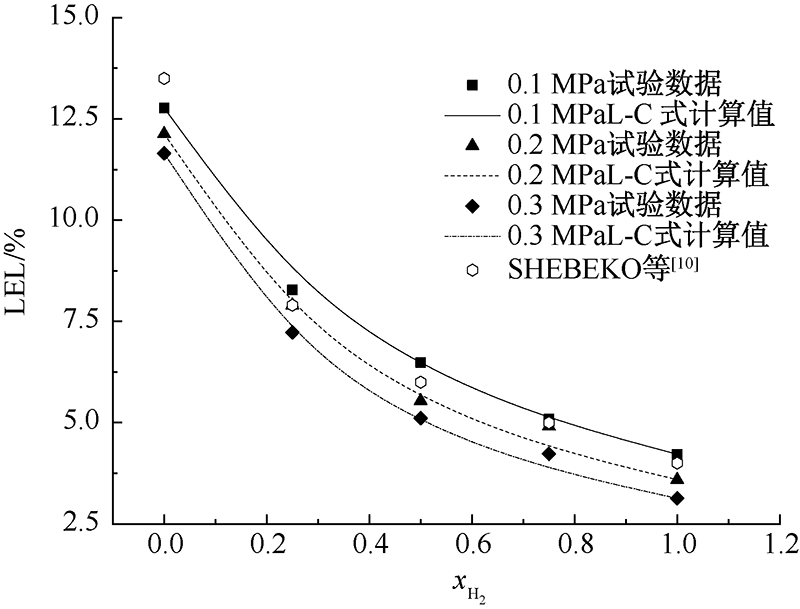
SHEBEKO Y N, FAN W, BOLODIAN I A. An analytical evaluation of flammability limits of gaseous mixtures of combustible-oxidizer-diluent[J]. Fire Safety Journal, 2002, 37(6):549-568.
doi: 10.1016/S0379-7112(02)00007-3
|
| [11] |
WANG Pengxiang, ZHAO Yijun, CHEN Yilun, et al. Study on the lower flammability limit of H2/CO in O2/H2O environment[J]. International Journal of Hydrogen Energy, 2017, 42(16):11926-11 936.
|
| [12] |
MIAO Haiyan, LIN Lu, HUANG Zuohua. Flammability limits of hydrogen-enriched natural gas[J]. International Journal of Hydrogen Energy, 2011, 36:6937-6947.
doi: 10.1016/j.ijhydene.2011.02.126
|
| [13] |
李国梁, 蒋军成, 潘勇. 基于绝热火焰温度混合气体爆炸下限的预测[J]. 中国安全科学学报, 2011, 21(7):57-61.
|
|
LI Guoliang, JIANG Juncheng, PAN Yong. Prediction on lower explosive limit of mixed gas based on calculated adiabatic flame temperature[J]. China Safety Science Journal, 2011, 21(7):57-61.
|
| [14] |
KONDO S, TAKAHASHI A, TAKIZAWA K, et al. On the pressure dependence of flammability limits of CH2=CFCF3,CH2F2 and methane[J]. Fire Safety Journal, 2011, 46(5):289-293.
doi: 10.1016/j.firesaf.2011.03.005
|