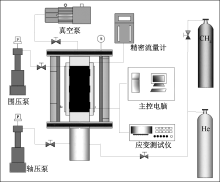
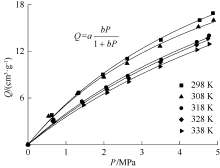
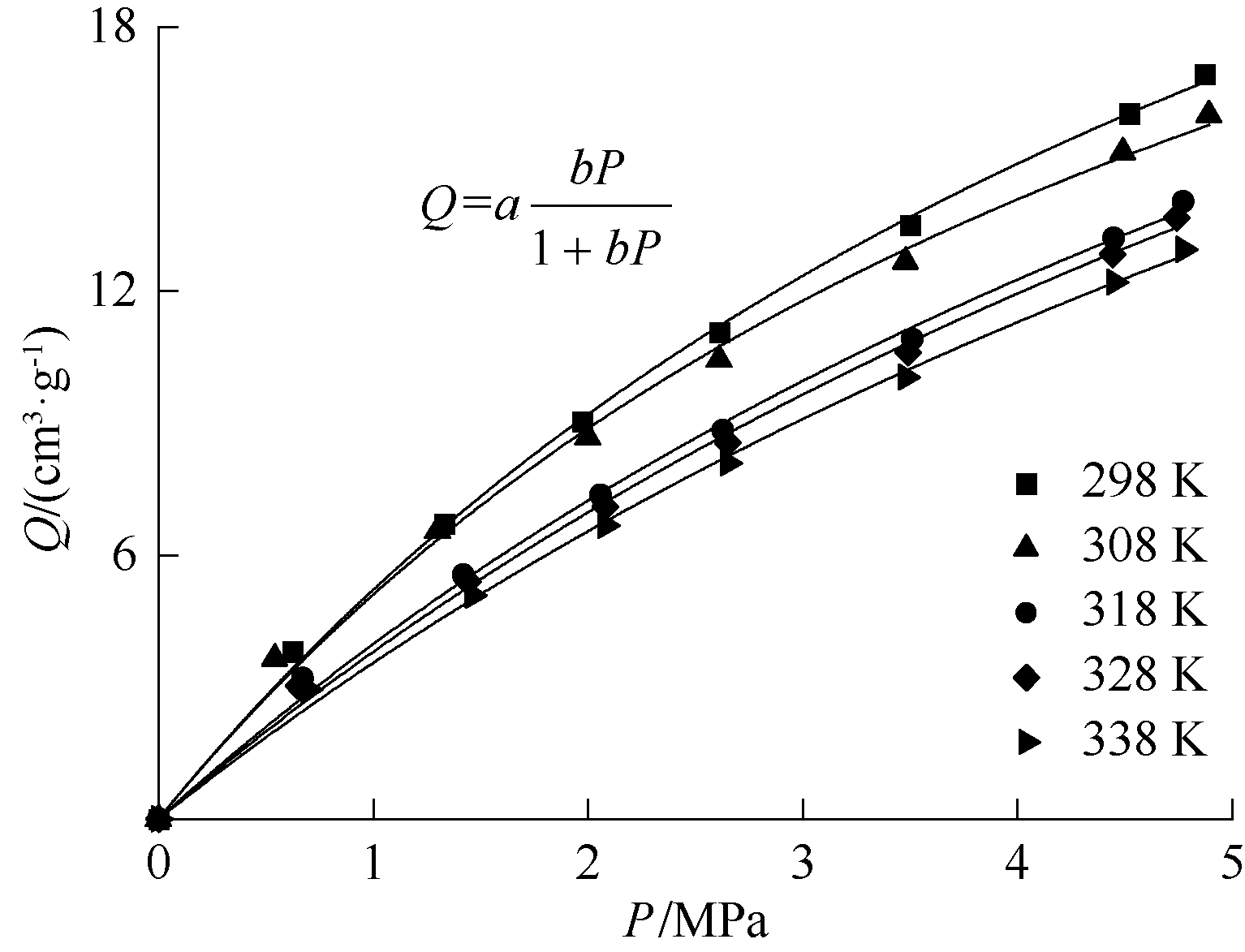
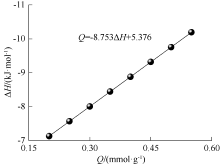
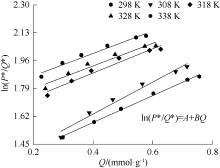
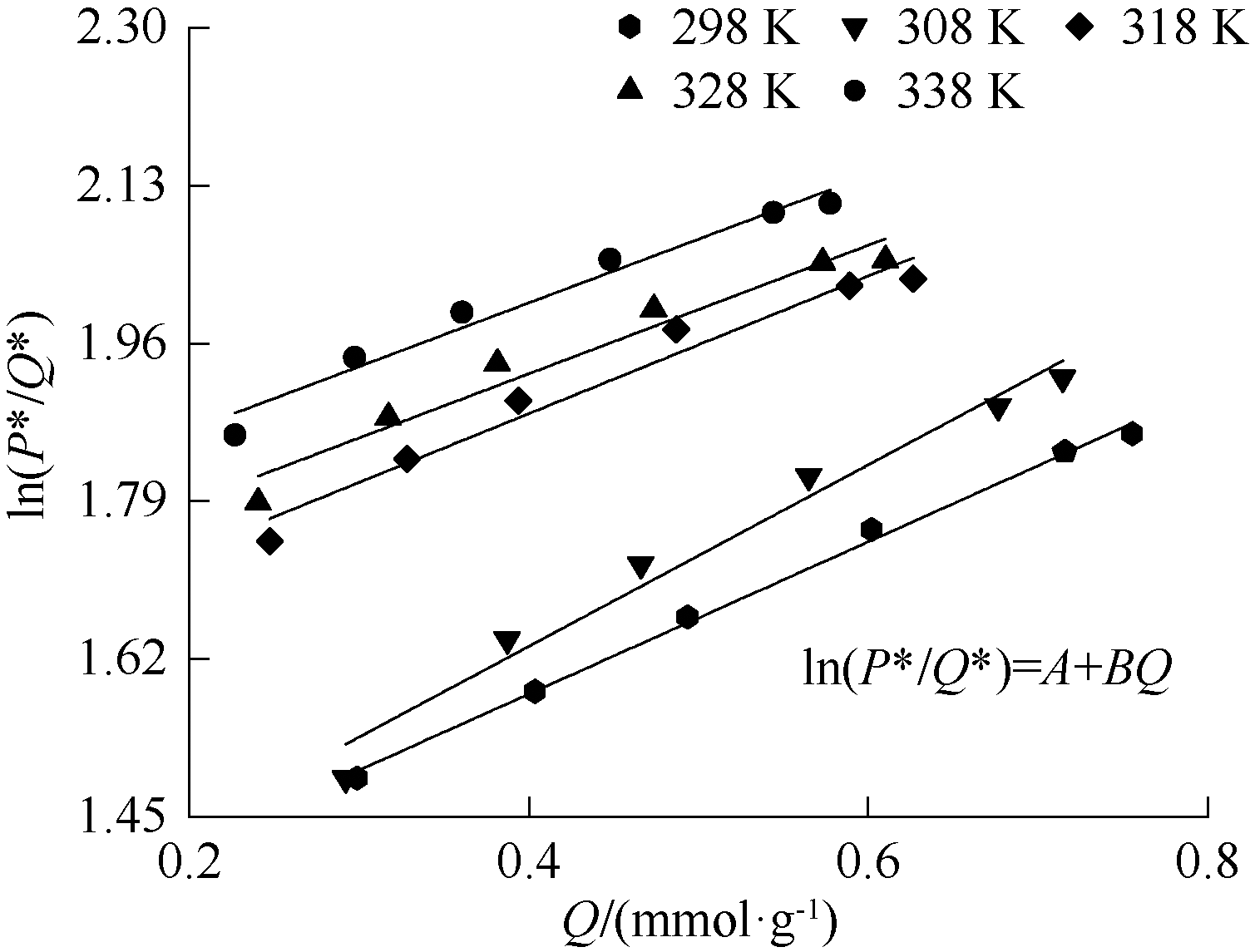
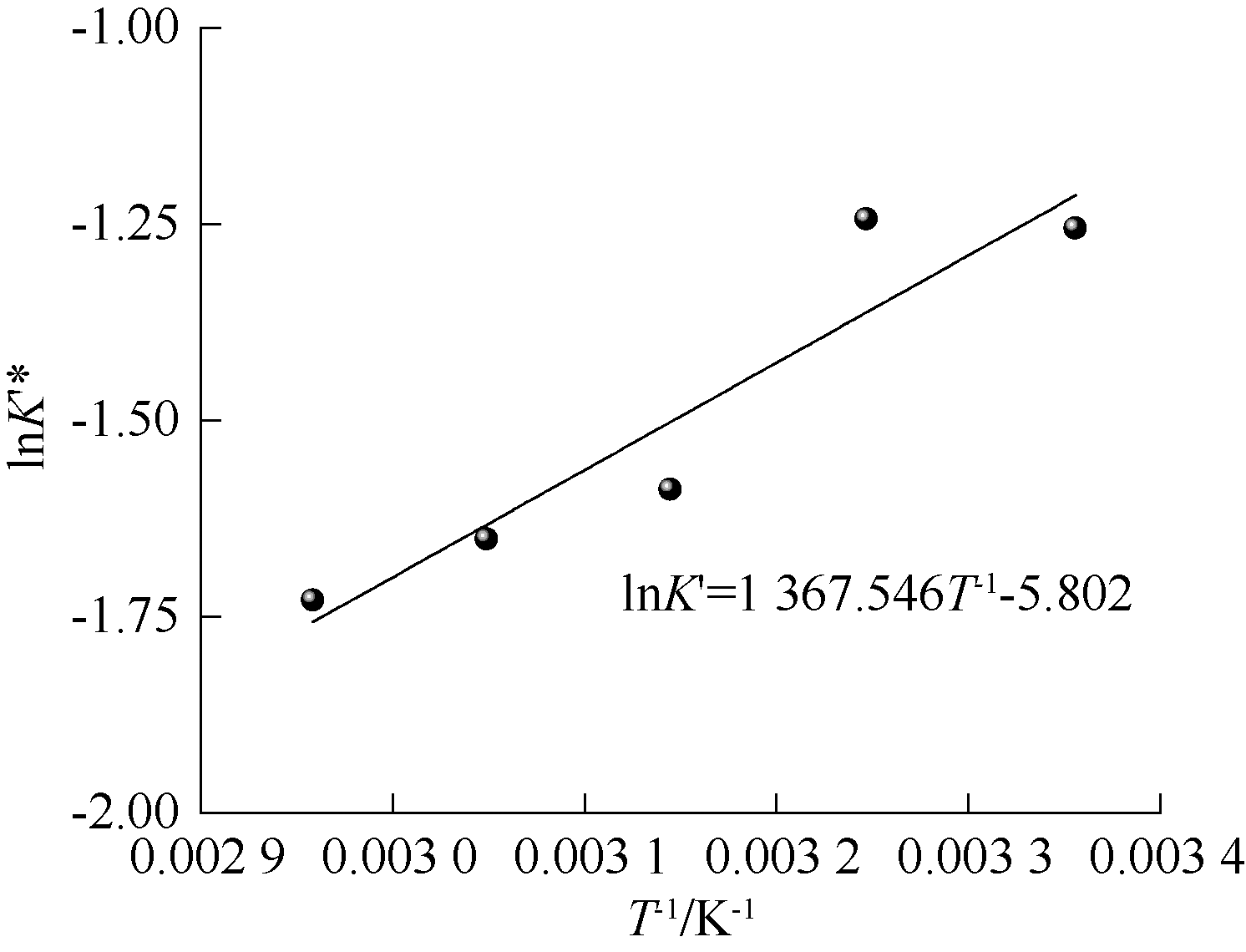
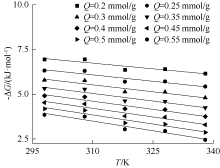
| [1] |
钱鸣高, 许家林, 王家臣. 再论煤炭的科学开采[J]. 煤炭学报, 2018, 43(1): 1-13.
|
|
QIAN Minggao, XU Jialin, WANG Jiachen. Further on the sustainable mining of coal[J]. Journal of China Coal Society, 2018, 43(1): 1-13.
|
| [2] |
张超林, 王恩元, 王奕博, 等. 近20年我国煤与瓦斯突出事故时空分布及防控建议[J]. 煤田地质与勘探, 2021, 49(4): 134-141.
|
|
ZHANG Chaolin, WANG Enyuan, WANG Yibo, et al. Spatial-temporal distribution of outburst accidents from 2001 to 2020 in China and suggestions for prevention and control[J]. Geology & Exploration, 2021, 49(4): 134-141.
|
| [3] |
祝捷, 张敏, 传李京, 等. 煤吸附/解吸瓦斯变形特征及孔隙性影响实验研究[J]. 岩石力学与工程学报, 2016, 35(增1): 2620-2626.
|
|
ZHU Jie, ZHANG Min, CHUAN Lijing, et al. Experimental study on coal strain induced by methane sorption/desorption and effect of pore features[J]. Chinese Journal of Rock Mechanics and Engineering, 2016, 35(S1): 2620-2626.
|
| [4] |
林海飞, 李树刚, 赵鹏翔, 等. 我国煤矿覆岩采动裂隙带卸压瓦斯抽采技术研究进展[J]. 煤炭科学技术, 2018, 46(1): 28-35.
|
|
LIN Haifei, LI Shugang, ZHAO Pengxiang, et al. Research progress on pressure released gas drainage technology of mining cracking zone in overburden strata of coal mine in China[J]. Coal Science and Technology, 2018, 46(1): 28-35.
|
| [5] |
张明杰, 杨明鑫, 闫江伟, 等. 超临界状态下中高阶变质煤吸附甲烷特性研究[J]. 中国安全科学学报, 2021, 31(8):53-61.
doi: 10.16265/j.cnki.issn1003-3033.2021.08.008
|
|
ZHANG Mingjie, YANG Mingxin, YAN Jiangwei, et al. Study on supercritical methane adsorption characteristics of coal with medium and high rank[J]. China Safety Science Journal, 2021, 31(8): 53-61.
doi: 10.16265/j.cnki.issn1003-3033.2021.08.008
|
| [6] |
DUAN Shuo, GU Min, TAO Mengeng, et al. Adsorption characteristics and thermodynamic property fields of methane and Sichuan Basin shales[J]. Adsorption, 2022, 28(1/2): 41-54.
doi: 10.1007/s10450-021-00352-6
|
| [7] |
张巨峰, 施式亮, 鲁义, 等. 矿井瓦斯与煤自燃共生灾害:耦合关系、致灾机制、防控技术[J]. 中国安全科学学报, 2020, 30(10): 149-155.
doi: 10.16265/j.cnki.issn 1003-3033.2020.10.021
|
|
ZHANG Jufeng, SHI Shiliang, LU Yi, et al. Symbiotic disasters of mine gas and coal spontaneous combustion: coupling relationship, disaster mechanism, prevention and control technology[J]. China Safety Science Journal, 2020, 30(10): 149-155.
doi: 10.16265/j.cnki.issn 1003-3033.2020.10.021
|
| [8] |
LI Xijian, LIN Baiquan, XU Hao. Monte Carlo simulation of methane molecule adsorption on coal with adsorption potential[J]. International Journal of Mining Science and Technology, 2014, 24(1): 17-22.
doi: 10.1016/j.ijmst.2013.12.004
|
| [9] |
聂百胜, 段三明. 煤吸附瓦斯的本质[J]. 太原理工大学学报, 1998, 29(4): 88-92.
|
|
NIE Baisheng, DUAN Sanming. The adsorption essence of gas on coal surface[J]. Journal of Taiyuan University of Technology, 1998, 29 (4): 88-92.
|
| [10] |
李树刚, 白杨, 林海飞, 等.CH4, CO2和N2多组分气体在煤分子中吸附热力学特性的分子模拟[J]. 煤炭学报, 2018, 43(9): 2476-2483.
|
|
LI Shugang, BAI Yang, LIN Haifei, et al. Molecular simulation of adsorption thermodynamics of multicomponent gas in coal[J]. Journal of China Coal Society, 2018, 43(9): 2476-2483.
|
| [11] |
林海飞, 蔚文斌, 李树刚, 等. 煤体吸附CH4及CO2热力学特性试验研究[J]. 中国安全科学学报, 2018, 28(6): 129-134.
doi: 10.16265/j.cnki.issn1003-3033.2018.06.022
|
|
LIN Haifei, WEI Wenbin, LI Shugang, et al. Experimental study on thermodynamics characteristics of CH4 and CO2 adsorption on coal[J]. China Safety Science Journal, 2018, 28(6): 129-134.
doi: 10.16265/j.cnki.issn1003-3033.2018.06.022
|
| [12] |
周银波, 王思琪, 毛淑星, 等. 热效应对焦煤甲烷解吸迟滞特征的影响研究[J]. 中国安全生产科学技术, 2020, 16(11): 123-127.
|
|
ZHOU Yinbo, WANG Siqi, MAO Shuxing, et al. Study on influence of thermal effect on methane desorption hysteresis characteristics of coking coal[J]. Journal of Safety Science and Technology, 2020, 16(11): 123-127.
|
| [13] |
TANG Xu, RIPEPI N, STADIE N P, et al. Thermodynamic analysis of high pressure methane adsorption in Longmaxi shale[J]. Fuel, 2017, 193: 411-418.
doi: 10.1016/j.fuel.2016.12.047
|
| [14] |
白建平, 张典坤, 杨建强, 等. 寺河3号煤甲烷吸附解吸热力学特征[J]. 煤炭学报, 2014, 39(9): 1812-1819.
|
|
BAI Jianping, ZHANG Diankun, YANG Jianqiang, et al. Thermodynamic characteristics of adsorption-desorption of methane in coal seam 3 at Sihe coal mine[J]. Journal of China Coal Society, 2014, 39(9): 1812-1819.
|
| [15] |
WANG Zhaofeng, TANG Xu, YUE Gaowei, et al. Physical simulation of temperature influence on methane sorption and kinetics in coal: benefits of temperature under 273. 15 K[J]. Fuel, 2015, 158(2): 207-216.
doi: 10.1016/j.fuel.2015.05.011
|
| [16] |
李树刚, 白杨, 林海飞, 等. 温度对煤吸附瓦斯的动力学特性影响实验研究[J]. 西安科技大学学报, 2018, 38(2): 181-186,272.
|
|
LI Shugang, BAI Yang, LIN Haifei, et al. Experimental study on the effect of temperature on the kinetics characteristics of gas adsorption on coal[J]. Journal of Xi'an University of Science and Technology, 2018, 38(2): 181-186,272.
|
| [17] |
ZANG Jie, WANG Kai, ZHAO Yixin. Evaluation of gas sorption induced internal swelling in coal[J]. Fuel, 2015, 143: 165-172.
doi: 10.1016/j.fuel.2014.11.007
|
| [18] |
赵东, 冯增朝, 赵阳升. 基于吸附动力学理论分析水分对煤体吸附特性的影响[J]. 煤炭学报, 2014, 39(3): 518-523.
|
|
ZHAO Dong, FENG Zengchao, ZHAO Yangsheng, et al. Effects of liquid water on coalbed methane adsorption characteristics based on the adsorption kinetic theory[J]. Journal of China Coal Society, 2014, 39(3): 518-523.
|
| [19] |
李育辉, 崔永君, 钟玲文, 等. 煤基质中甲烷扩散动力学特性研究[J]. 煤田地质与勘探, 2005, 33(6): 31-34.
|
|
LI Yuhui, CUI Yongjun, ZHONG Lingwen, et al. Study on dynamic diffusion characteristics of methane in coal matrix[J]. Coal Geology &Exploration, 2005, 33(6): 31-34.
|
| [20] |
LANGMUIR I. The adsorption of gases on plane surfaces of glass, mica and platinum[J]. Journal of Chemical Physics, 1918, 40(12): 1361-1403.
|
| [21] |
张翔, 陶云奇. 不同温度条件下煤对瓦斯的等温吸附实验研究[J]. 煤炭工程, 2011, 43(4): 87-89.
|
|
ZHANG Xiang, TAO Yunqi. Experiment study on gas isothermal adsorption from coal under different temperature conditions[J]. Coal Engineering, 2011, 43(4): 87-89.
|
| [22] |
刘志祥, 冯增朝. 煤体对瓦斯吸附热的理论研究[J]. 煤炭学报, 2012, 37(4): 647-653.
|
|
LIU Zhixiang, FENG Zengchao. Theoretical study on adsorption heat of methane in coal[J]. Journal of China Coal Society, 2012, 37(4): 647-653.
|
| [23] |
RAMIREZ P A J, BULNES F. Differential heat of adsorption in the presence of an order-disorder phase transition[J]. Physica A: Statistical Mechanics and its Applications, 2000, 283(1/2): 198-203.
doi: 10.1016/S0378-4371(00)00152-7
|
| [24] |
郑青榕, BIRKETT G, DO D D. 甲烷在活性炭上吸附的实验及理论分析[J]. 天然气化工(C1化学与化工), 2009, 34(1): 41-45.
|
|
ZHENG Qingrong, BIRKETT G, DO D D. Theoretical and experimental analysis of methane adsorption on activated carbon[J]. Natural Gas Chemical Industry, 2009, 34(1): 41-45.
|
| [25] |
HELFFERICH F G. Principles of adsorption & adsorption processes, by D. M. Ruthven, John Wiley & Sons, 1984, xxiv + 433 pp[J]. AIChE Journal, 1985, 31(3): 523-524.
|
| [26] |
ONSALVO M A, SHAPIRO A A. Study of high-pressure adsorption from supercritical fluids by the potential theory[J]. Fluid Phase Equilibria, 2009, 283(1): 56-64.
doi: 10.1016/j.fluid.2009.05.015
|
| [27] |
DUBININM M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces[J]. Chemical Reviews, 1960, 60(2): 235-241.
doi: 10.1021/cr60204a006
|
| [28] |
MATHIAS P M, O'CONNELL J P. The Gibbs-Helmholtz equation and the thermodynamic consistency of chemical absorption data[J]. Industrial & Engineering Chemistry Research, 2012, 51(13): 5090-5097.
doi: 10.1021/ie202668k
|
| [29] |
LAGERGREN S. About the theory of so-called adsorption of soluble substances[J]. Kungliga Svenska Vetenskapsakademiens Handlingar, 1898, 24 (4): 1-39.
|
| [30] |
HO Y S, MCKAY G. Pseudo-second order model for sorption processes[J]. Process Biochemist, 1999, 34(5): 451-465.
|