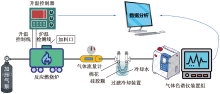
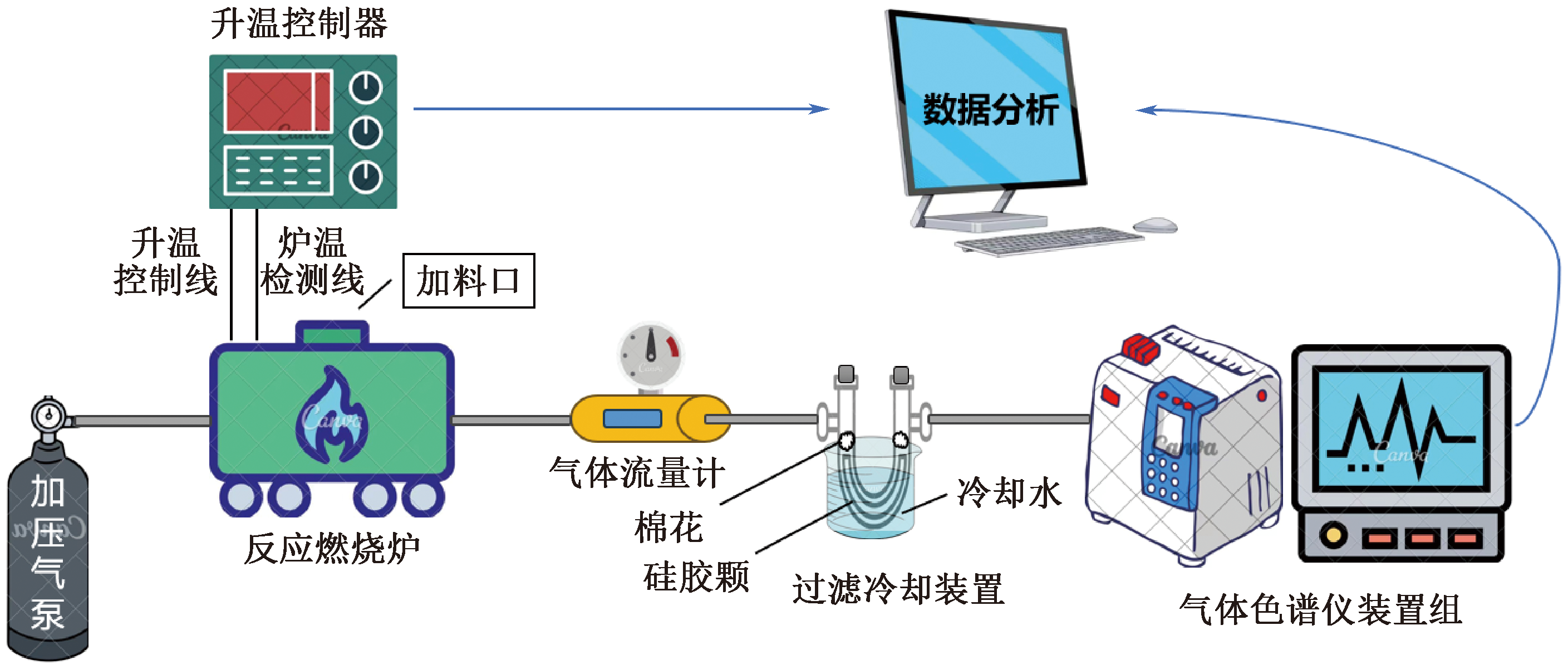
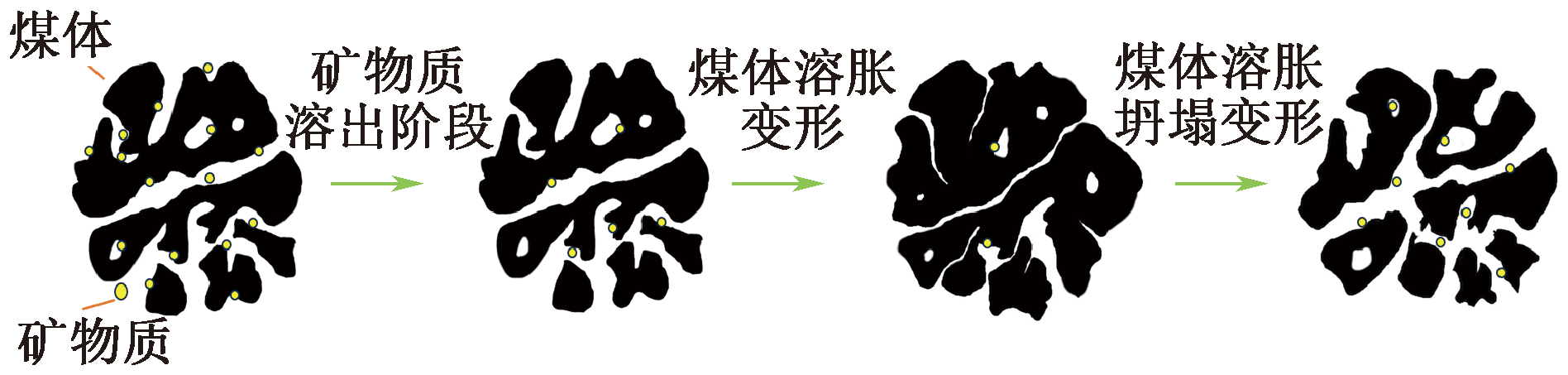
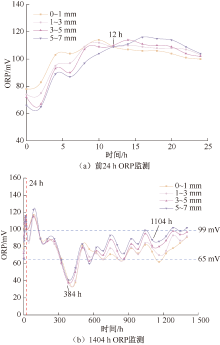
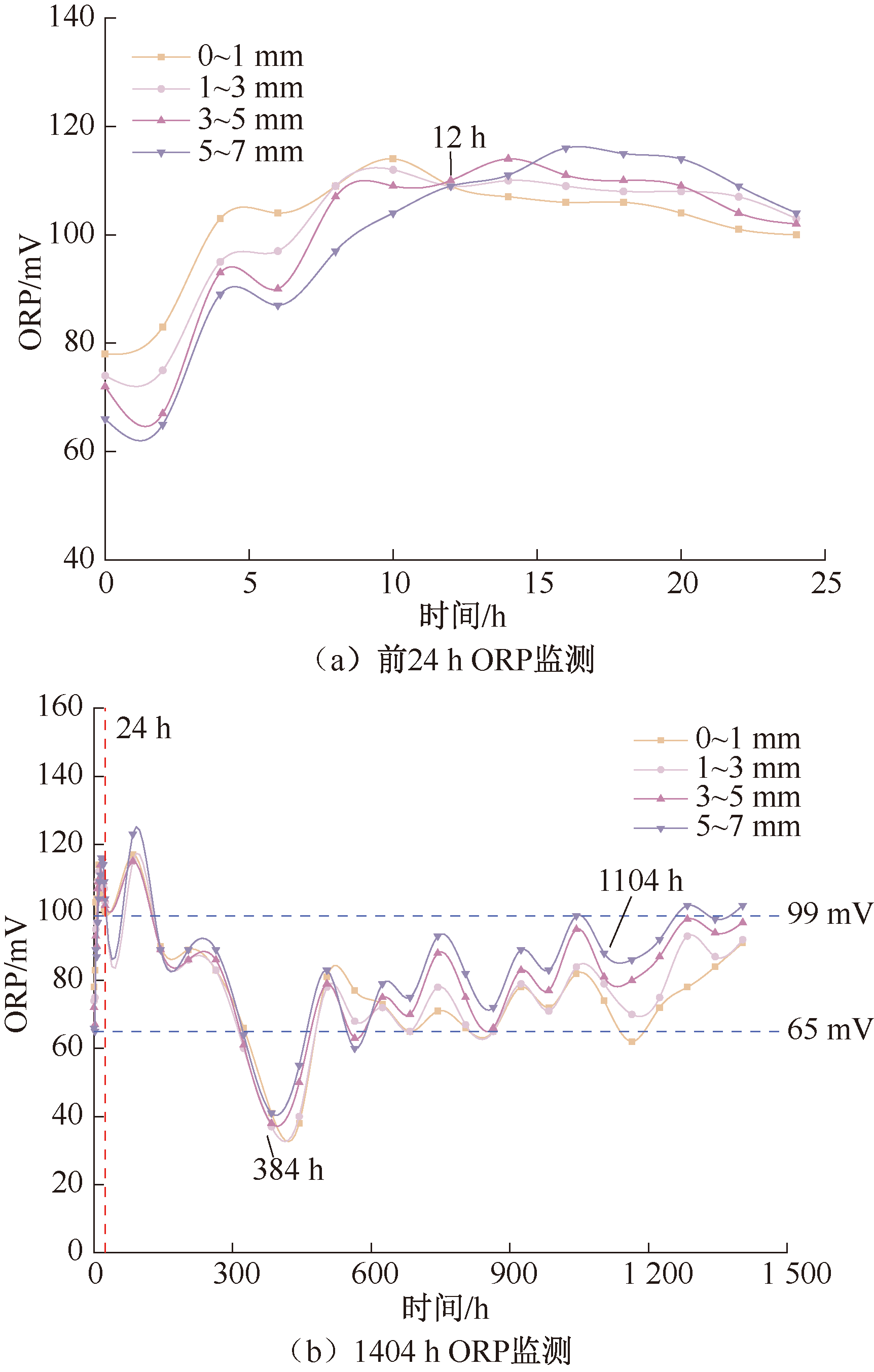
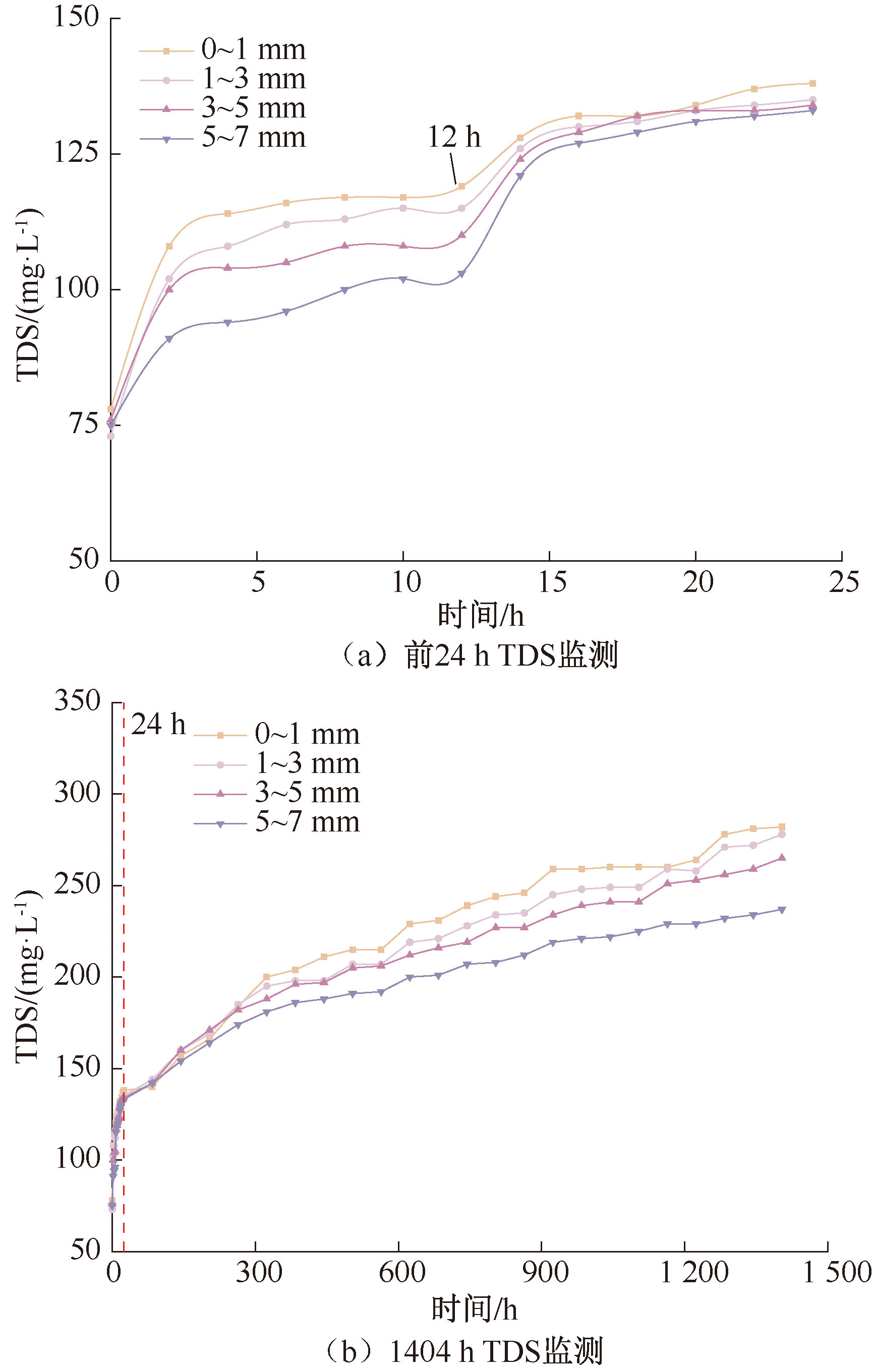
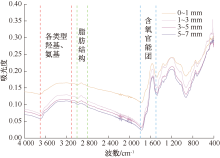
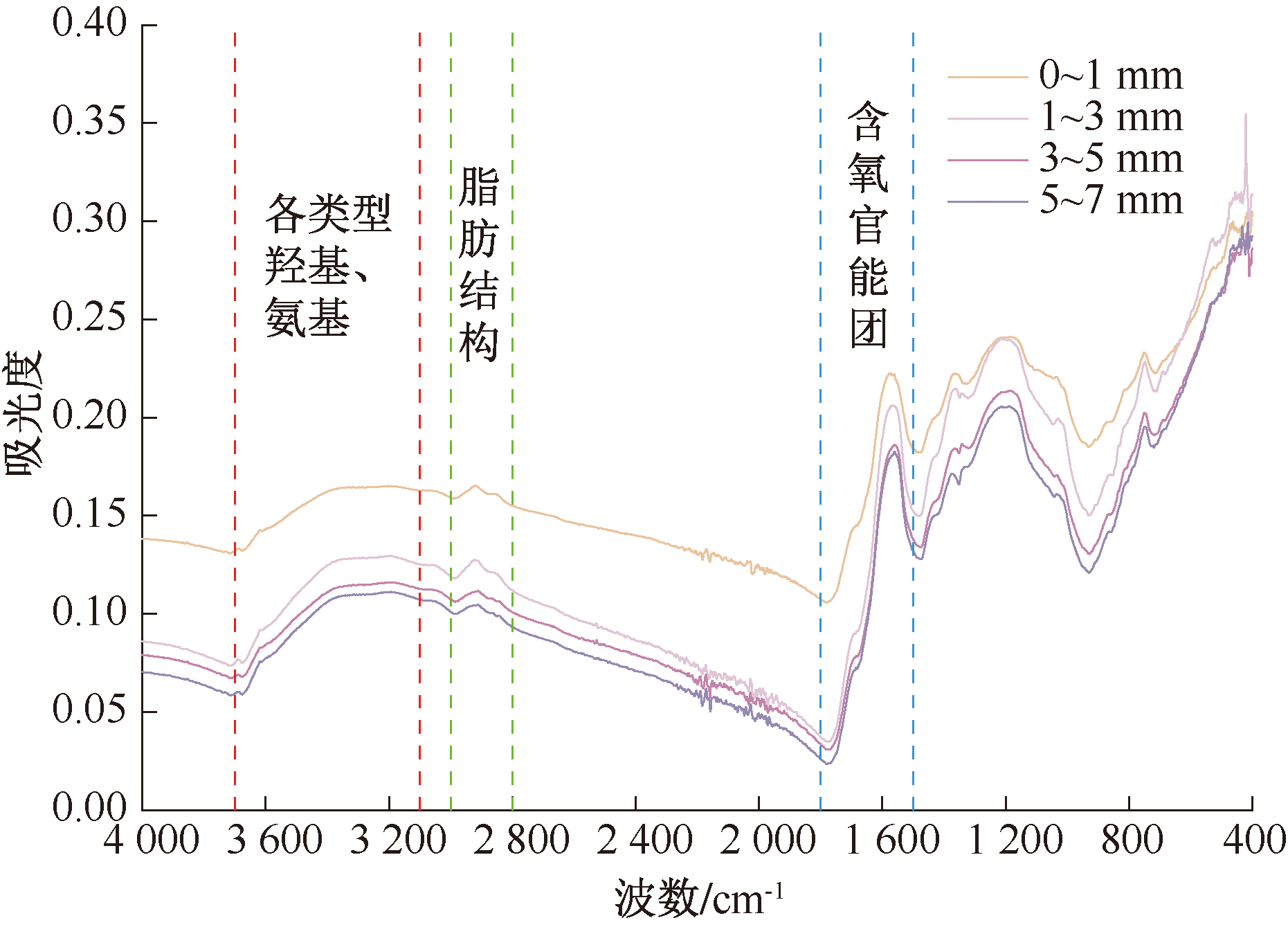
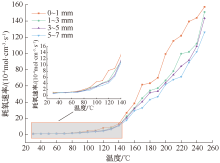
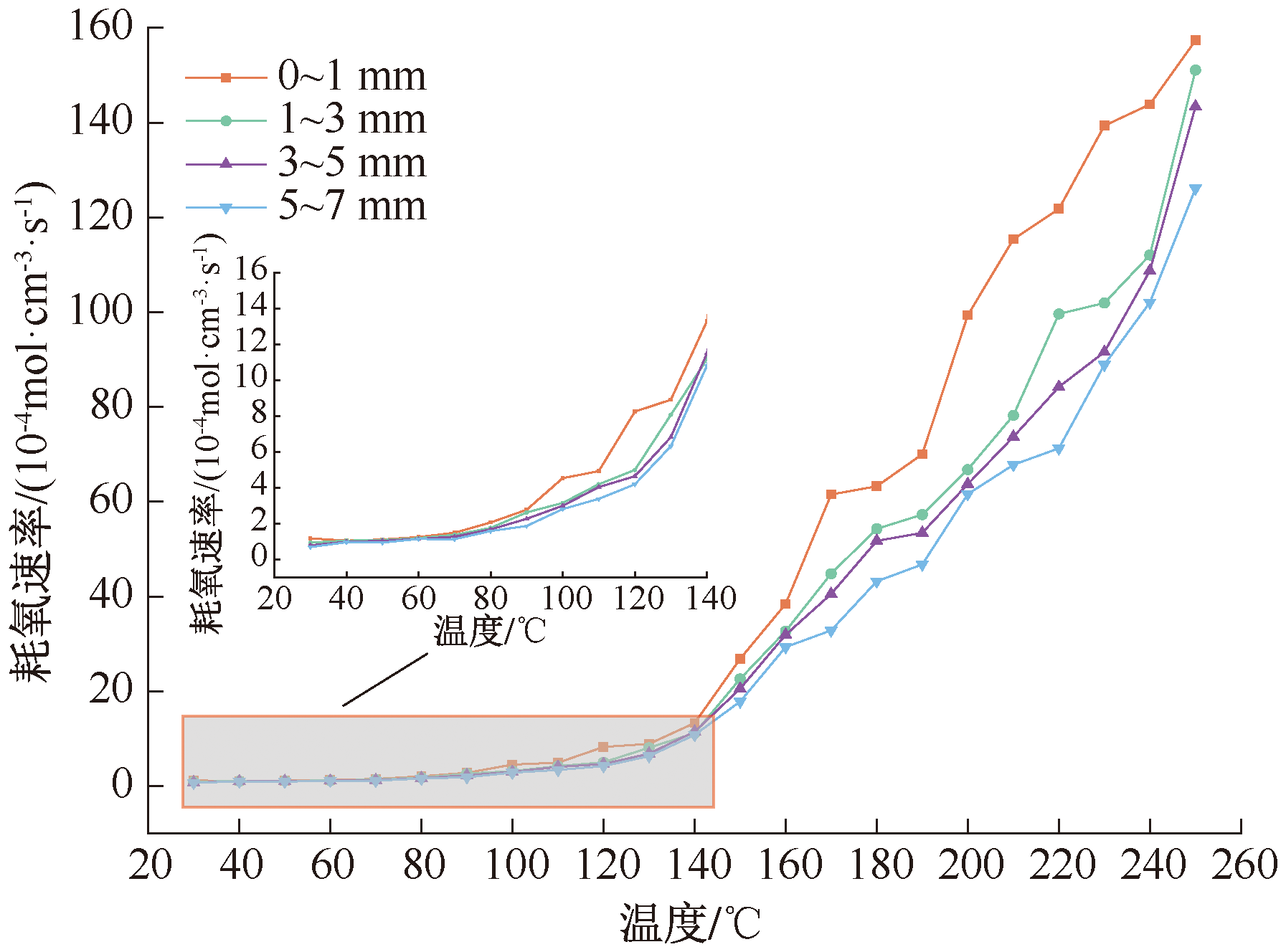
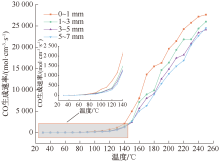
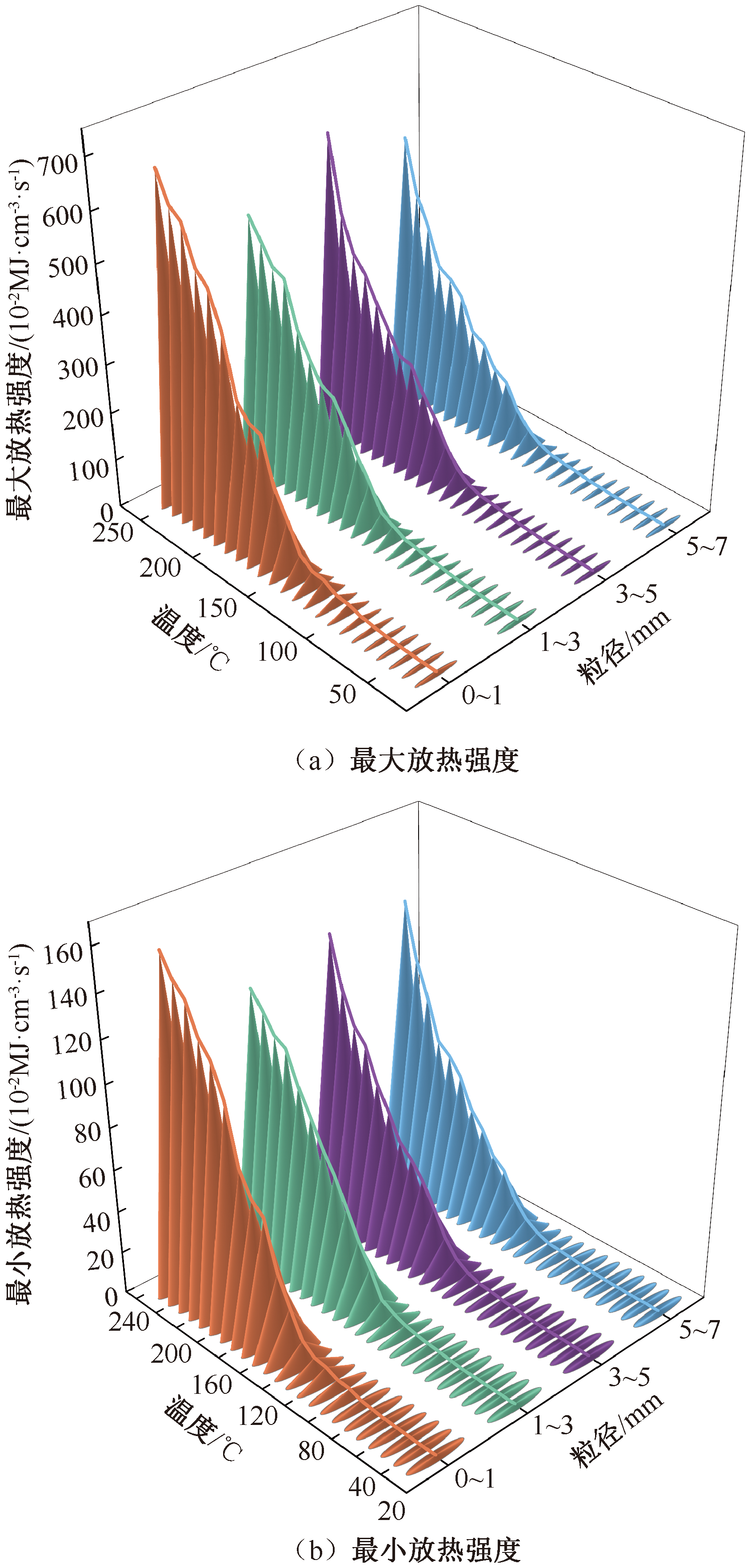
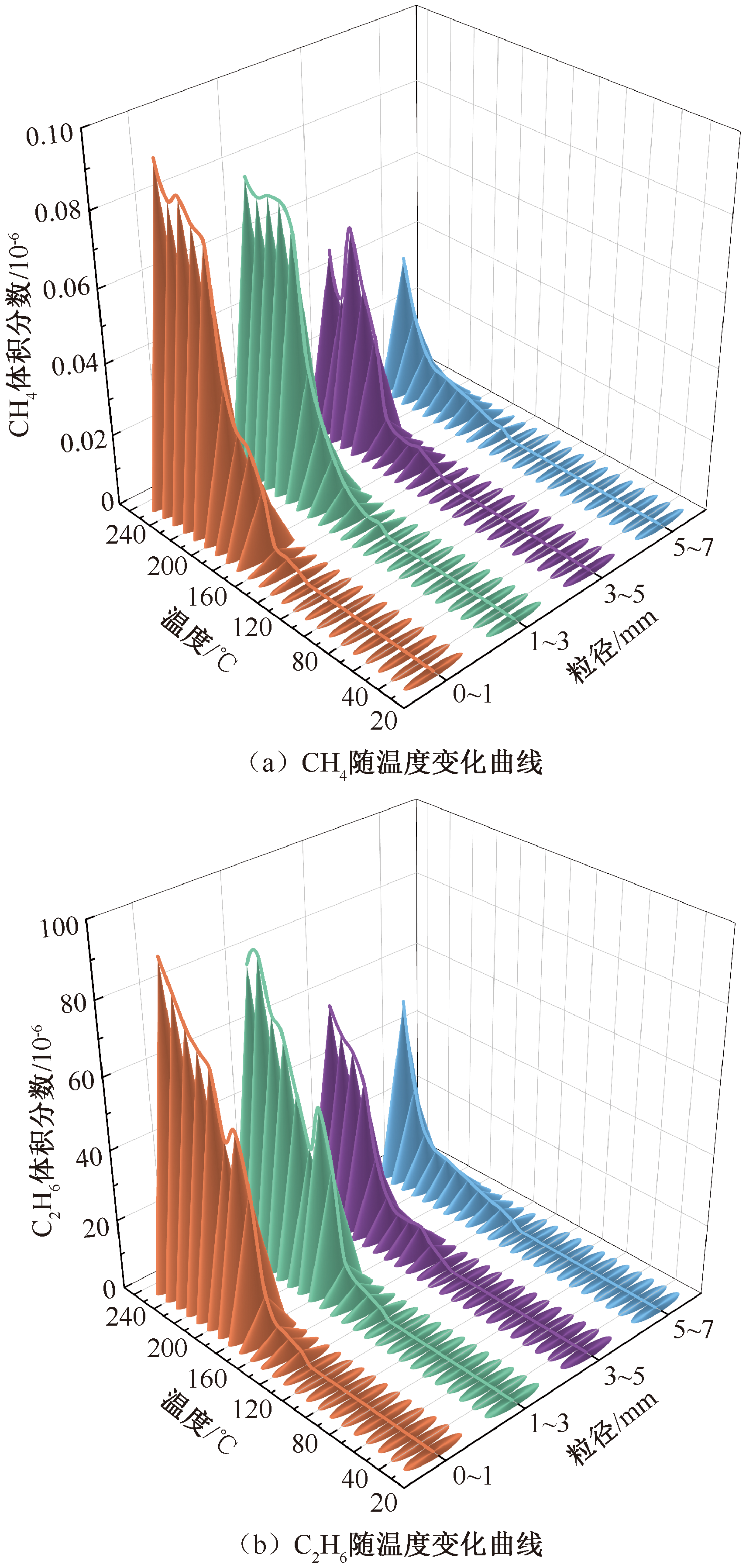
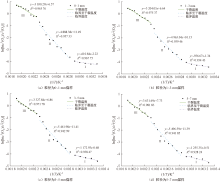
| [1] |
JIANG Xiaoyuan, YANG Shengqiang, ZHOU Buzhuang, et al. The auto-oxidation characteristic of coal at different stages of the low-temperature oxidation process[J]. Fuel, 2023,352: DOI: 10.1016/j.fuel.2023.129130.
|
| [2] |
LU Hao, LI Jinliang, LU Wei, et al. Variation laws of CO 2/CO and influence of key active groups on it during low-temperature oxidation of coal[J]. Fuel, 2023,339: DOI: 10.1016/j.fuel.2023.127415.
|
| [3] |
秦波涛, 仲晓星, 王德明, 等. 煤自燃过程特性及防治技术研究进展[J]. 煤炭科学技术, 2021, 49(1): 66-99.
|
|
QIN Botao, ZHONG Xiaoxing, WANG Deming, et al. Research progress of coal spontaneous combustion process characteristics and prevention technology[J]. Coal Science and Technology, 2021, 49(1): 66-99.
|
| [4] |
DENG Jun, QU Gaoyang, REN Shuaijing, et al. Effect of water soaking and air drying on the thermal effect and heat transfer characteristics of coal oxidation at the low-temperature oxidation stage[J]. Energy, 2024,288: DOI: 10.1080/10407782.2024.2384617.
|
| [5] |
ZHAO Jingwen, WANG Wencai, FU Peng, et al. Evaluation of the spontaneous combustion of soaked coal based on a temperature-programmed test system and in-situ FTIR[J]. Fuel, 2021,294: DOI: 10.1016/j.fuel.2021.110878.
|
| [6] |
LI Lin, LIU Tiantian, CHEN Xiangjun, et al. Effect of water immersion pressure on oxidation characteristics and spontaneous combustion characteristics of long-flame coal[J]. Energy, 2024,291: DOI: 10.1016/j.energy.2024.130331.
|
| [7] |
易欣, 葛龙, 张少航, 等. 基于指标气体法对水浸煤的氧化特性研究[J]. 煤炭科学技术, 2023, 51(3): 130-136.
|
|
YI Xin, GE Long, ZHANG Shaohang, et al. Research on oxidation characteristics of aqueous coal based on index gas method[J]. Coal Science and Technology, 2023, 51(3): 130-136.
|
| [8] |
GUO Shengli, YUAN Shujie, GENG Weile, et al. Erosion effect on microstructure change and oxidation behavior of long-flame coal under different pH conditions[J]. Fuel, 2021,293: DOI: 10.1134/S0010508216010093.
|
| [9] |
LUO Jinzhi, CAI Yanyan, TANG Hao, et al. Mechanism of the pore and molecular structure evolution of coal exposed to acid mine drainage (AMD)[J]. Science of The Total Environment, 2024,906: DOI: 10.1016/j.scitotenv.2023.167836.
|
| [10] |
肖知国, 郝梅, 唐志昊, 等. 酸化-气润湿反转对煤吸附特性影响的实验研究[J]. 煤炭科学技术, 2024, 52(增2): 79-87.
|
|
XIAO Zhiguo, HAO Mei, TANG Zhihao, et al. Experimental study on adsorption characteristics of coal by acidification-gas wettability alteration[J]. Coal Science and Technology, 2024, 52(S2): 79-87.
|
| [11] |
马东, 解庆典, 赵志强, 等. 孟巴矿高地温环境煤孔隙及氧化动力学特征[J]. 中国安全科学学报, 2024, 34(8): 162-169.
doi: 10.16265/j.cnki.issn1003-3033.2024.08.0061
|
|
MA Dong, XIE Qingdian, ZHAO Zhiqiang, et al. Characteristics of high temperature environment on coal pore structure and oxidation dynamics of Barapukuria coal mine in Bangladesh[J]. China Safety Science Journal, 2024, 34(8): 162-169.
doi: 10.16265/j.cnki.issn1003-3033.2024.08.0061
|
| [12] |
周琛鸿, 李绪萍, 张靖, 等. 不同浸水率煤矸石浸水复干氧化特性研究[J]. 煤炭科学技术, 2024, 52(增1): 107-115.
|
|
ZHOU Chenhong, LI Xuping, ZHANG Jing, et al. Study on oxidation characteristics of coal gangue with different moisture content under water immersion drying[J]. Coal Science and Technology, 2024, 52(S1): 107-115.
|
| [13] |
张小东, 亢红东, 李冰辉, 等. 不同溶剂预处理煤在ScCO2作用下的谱学差异及其机制[J]. 光谱学与光谱分析, 2024, 44(9): 2657-2666.
|
|
ZHANG Xiaodong, KANG Hongdong, LI Binghui, et al. Spectroscopic differences in different solvent pretreated coals in the presence of ScCO2 and their mechanisms[J]. Spectroscopy and Spectral Analysis, 2024, 44(9): 2657-2666.
|
| [14] |
杨耀辉, 申全军, 丛波日, 等. 煤基硬质改性沥青改性机理初探[J]. 洁净煤技术, 2024, 30(增2): 135-140.
|
|
YANG Yaohui, SHEN Quanjun, CONG Bori, et al. Study on modification mechanism of coal-based hard modified asphalt[J]. Clean Coal Technology, 2024, 30(S2): 135-140.
|
| [15] |
田泽奇, 王志勇, 姚建国, 等. 岩浆接触带高变质煤化学结构FTIR定量表征[J]. 光谱学与光谱分析, 2023, 43(9): 2747-2754.
|
|
TIAN Zeqi, WANG Zhiyong, YAO Jianguo, et al. Quantitative FTIR characterization of chemical structures of highly metamorphic coals in a magma contact zone[J]. Spectroscopy and Spectral Analysis, 2023, 43(9): 2747-2754.
|
| [16] |
叶正亮, 郭曦蔓, 尚博, 等. 煤变质程度对微观结构与热解参数的影响及关联性分析[J]. 中国安全科学学报, 2025, 35(2): 57-65.
doi: 10.16265/j.cnki.issn1003-3033.2025.02.0698
|
|
YE Zhengliang, GUO Ximan, SHANG Bo, et al. Influence of coal metamorphism on microstructure and pyrolysis parameters: a correlation analysis[J]. China Safety Science Journal, 2025, 35(2): 57-65.
doi: 10.16265/j.cnki.issn1003-3033.2025.02.0698
|
| [17] |
张靖, 李天宇, 李绪萍, 等. 不同粒径煤矸石二次氧化指标气体产生规律研究[J]. 中国安全生产科学技术, 2024, 20(10): 94-100.
|
|
ZHANG Jing, LI Tianyu, LI Xuping, et al. Study on generation law of index gases for secondary oxidation of coal gangue with different particle sizes[J]. Journal of Safety Science and Technology, 2024, 20(10): 94-100.
|
| [18] |
丁徐琴, 张雷林. 氧气体积分数对煤自燃特性影响的实验研究[J]. 煤矿安全, 2023, 54(7): 156-162.
|
|
DING Xuqin, ZHANG Leilin. Experimental study on effect of oxygen volume fraction on spontaneous combustion characteristics of coal[J]. Safety in Coal Mines, 2023, 54(7): 156-162.
|
| [19] |
高飞, 白企慧, 贾喆, 等. 基于量子化学计算的煤低温氧化放热强度[J]. 煤炭学报, 2023, 48(9): 3428-3440.
|
|
GAO Fei, BAI Qihui, JIA Zhe, et al. Exothermicity of coal during low temperature oxidation process based onquantum chemical calculation[J]. Journal of China Coal Society, 2023, 48(9): 3428-3440.
|
| [20] |
潘荣锟, 胡代民, 贾海林, 等. 深部开采高温热液侵蚀煤自燃特性[J]. 煤炭学报, 2024, 49(4): 1906-1916.
|
|
PAN Rongkun, HU Daimin, JIA Hailiin, et al. Spontaneous combustion characteristics of hydrothermal eroded coal in deep mining[J]. Journal of China Coal Society, 2024, 49(4): 1906-1916.
|
| [21] |
李增华, 苗国栋. 煤自燃大分子量气态产物生成规律研究[J]. 中国矿业大学学报, 2023, 52(6): 1119-1128.
|
|
LI Zenghua, MIAO Guodong. Study of the emission law of higher-molecular-weight gases during coal spontaneous combustion[J]. Journal of China University of Mining & Technology, 2023, 52(6): 1119-1128.
|
| [22] |
YI Xin, ZHANG Min, DENG Jun, et al. Effects on environmental conditions and limiting parameters for spontaneous combustion of residual coal in underground goaf[J]. Process Safety and Environmental Protection, 2024,187: DOI: 10.1016/j.psep.2024.05.081.
|
| [23] |
闫国锋, 黄兴利, 闫振国. 预氧化煤低温氧化放热和动力学特性研究[J]. 工矿自动化, 2022, 48(7): 135-141.
|
|
YAN Guofeng, HUANG Xingli, YAN Zhenguo, et al. Research on exothermic and kinetic characteristics of low-temperature oxidation of preoxidized coal[J]. Journal of Mine Automation, 2022, 48(7): 135-141.
|
| [24] |
LI Purui, YANG Yongliang, ZHAO Xiaohao, et al. Spontaneous combustion and oxidation kinetic characteristics of alkaline-water-immersed coal[J]. Energy, 2023,263: DOI: 10.1016/j.tca.2021.178914.
|
| [25] |
郭志国, 张俊, 郑彪华, 等. 预氧化煤复燃行为演化特征及微观动力学机制[J]. 安全与环境学报, 2023, 23(8): 2644-2652.
|
|
GUO Zhiguo, ZHANG Jun, ZHENG Biaohua, et al. Evolution characteristics and microscopic dynamics mechanism of recrudescence behaviors of pre-oxidized coal[J]. Journal of Safety and Environment, 2023, 23(8): 2644-2652.
|