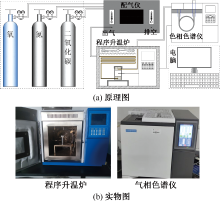
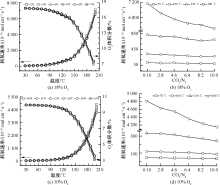
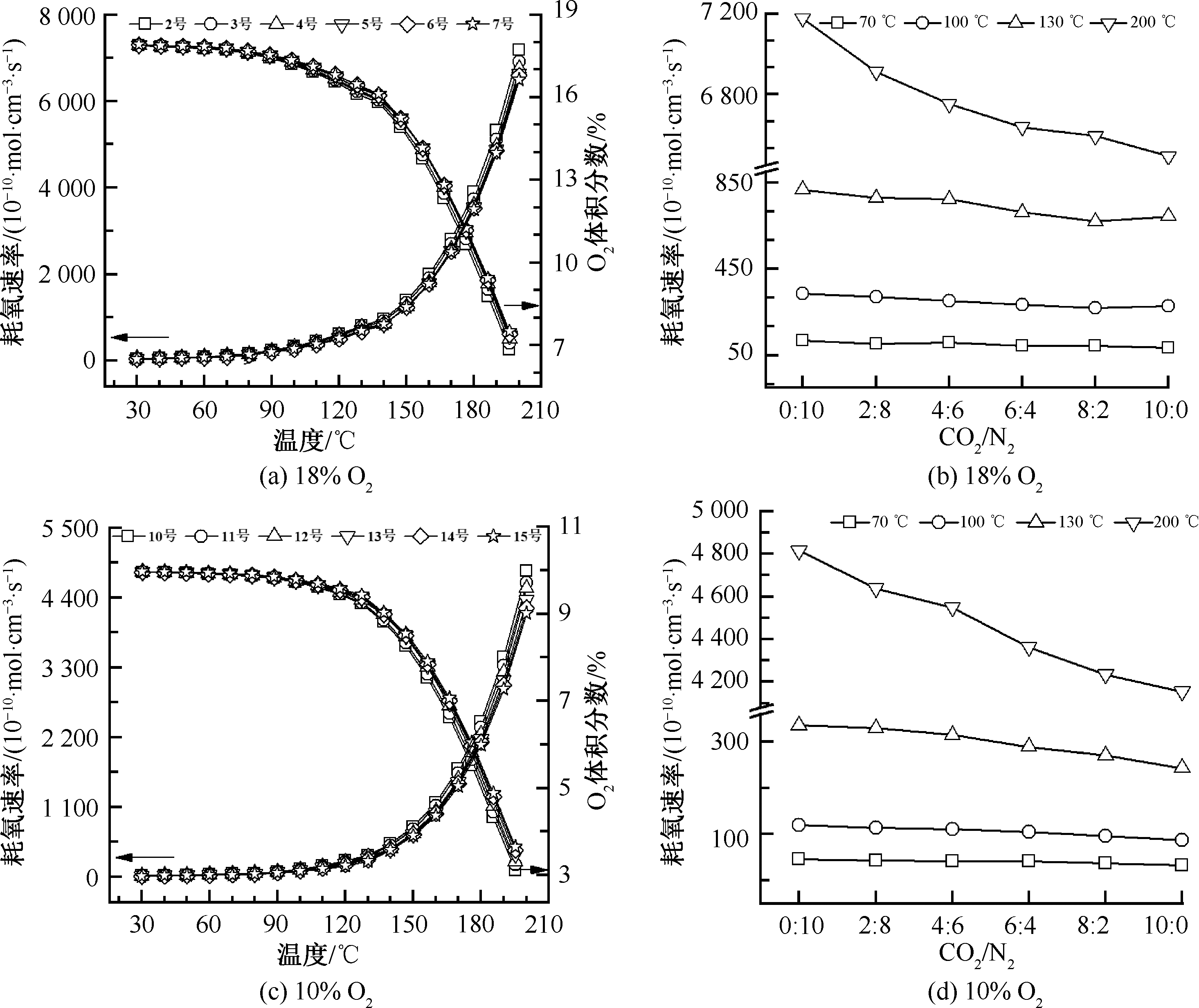
| [1] |
胡振琪, 肖武, 赵艳玲. 再论煤矿区生态环境“边采边复”[J]. 煤炭学报, 2020, 45(1):351-359.
|
|
HU Zhenqi, XIAO Wu, ZHAO Yanling. Re-discussion on coal mine eco-environment concurrent mining and reclamation[J]. Journal of China Coal Society, 2020, 45(1):351-359.
|
| [2] |
马东, 解庆典, 赵志强, 等. 孟巴矿高地温环境煤孔隙及氧化动力学特征[J]. 中国安全科学学报, 2024, 34(8):162-169.
doi: 10.16265/j.cnki.issn1003-3033.2024.08.0061
|
|
MA Dong, XIE Qingdian, ZHAO Zhiqiang, et al. Characteristics of high temperature environment on coal pore structure and oxidation dynamics of Barapukuria coal mine in Bangladesh[J]. China Safety Science Journal, 2024, 34(8):162-169.
doi: 10.16265/j.cnki.issn1003-3033.2024.08.0061
|
| [3] |
XI Zhilin, SUN Xutong. Effectiveness of thermoplastic powder to retard self-heating and spontaneous combustion of coal[J]. Combustion Science and Technology, 2016, 188(8):1331-1344.
|
| [4] |
张玉涛, 郭强, 张园勃, 等. 基于相关系数法的煤自燃危险性关联分析及预测[J]. 中国安全科学学报, 2024, 34(1):125-132.
doi: 10.16265/j.cnki.issn1003-3033.2024.01.0774
|
|
ZHANG Yutao, GUO Qiang, ZHANG Yuanbo, et al. Correlation analysis and prediction of coal spontaneous combustion risk based on correlation coefficient method[J]. China Safety Science Journal, 2024, 34(1):125-132.
doi: 10.16265/j.cnki.issn1003-3033.2024.01.0774
|
| [5] |
吴春雷. CO2-N2混合惰气的惰化特性及工程应用研究[D]. 徐州: 中国矿业大学, 2024.
|
|
WU Chunlei. Study on inerting characteristics and engineering applications of CO2-N2 mixed inert gas[D]. Xuzhou: China University of Mining and Technology, 2024.
|
| [6] |
TANG Li, QI Yudong, LI Ximing, et al. Coal fire prevention in large areas over long term with a composite inert gas—a case study in Tangkou coal mine, China[J]. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 2019, 46(1):1060-1070.
|
| [7] |
SHI Jingdong, SU Hetao, LI Yunzhuo, et al. Quantitative analysis of heat release during coal oxygen-lean combustion in a O2/CO2/N2 atmosphere by TG-DTG-DSC[J]. Scientific Reports, 2022, 12:DOI:10.1038/s41598-022-10752-5.
|
| [8] |
REN Lifeng, LI Qingwei, DENG Jun, et al. Inhibiting effect of CO2 on the oxidative combustion thermodynamics of coal[J]. RSC Advances, 2019, 9: 41 126-41 134.
|
| [9] |
WU Chunlei, LI Jia, ZHOU Fubao, et al. Experimental study on the competitive adsorption characteristics of N2, CO2 and O2 with various CO2/N2 blend ratios in coal[J]. International Journal of Hydrogen Energy, 2024,59:924-936.
|
| [10] |
WANG Changan, ZHANG Xiaoming, LIU Yinhe, et al. Pyrolysis and combustion characteristics of coals in oxyfuel combustion[J]. Applied Energy, 2012, 97: 264-273.
|
| [11] |
IRFAN M F, ARAMI-NIYA A, CHAKRABARTI M H, et al. Kinetics of gasification of coal, biomass and their blends in air (N2/O2) and different oxy-fuel (O2/CO2) atmospheres[J]. Energy, 2012,37:665-672.
|
| [12] |
ZHANG Sizong, WEN Zhi, WANG Gan, et al. Kinetic analyses of coke combustion and thermal decompositions of limestone and dolomite based on the sintering atmosphere[J]. Fuel, 2021,289:DOI: 10.1016/j.fuel.2020.119870.
|
| [13] |
WU Chunlei, GE Shaokun, LI Jia, et al. The effect of N 2/CO 2 blend ratios on the pyrolysis and combustion behaviors of coal particles: kinetic and thermodynamic analyses[J]. Journal of Loss Prevention in The Process Industries, 2023,84:DOI: 10.1016/j.jlp.2023.105120.
|
| [14] |
SU Hetao, KANG Ning, SHI Bobo, et al. Simultaneous thermal analysis on the dynamical oxygen-lean combustion behaviors of coal in a O2/N2/CO2 atmosphere[J]. Journal of the Energy Institute, 2021, 96: 128-139.
doi: 10.1016/j.joei.2021.03.003
|
| [15] |
郭志国, 王蓉, 张俊, 等. CO2防控氧化煤复燃效率的试验研究[J]. 矿业科学学报, 2021, 6(2):160-165.
|
|
GUO Zhiguo, WANG Rong, ZHANG Jun, et al. Experimental research on the fire-fighting effects of CO2 on the recrudescence process of oxidized coal[J]. Journal of Mining Science and Technology, 2021, 6(2):160-165.
|
| [16] |
National Institute of Science and Technology. NIST chemistry Webbook, SRD 69 [EB/OL]. (2023-01-01). https://webbook.nist.gov/chemistry/.
|