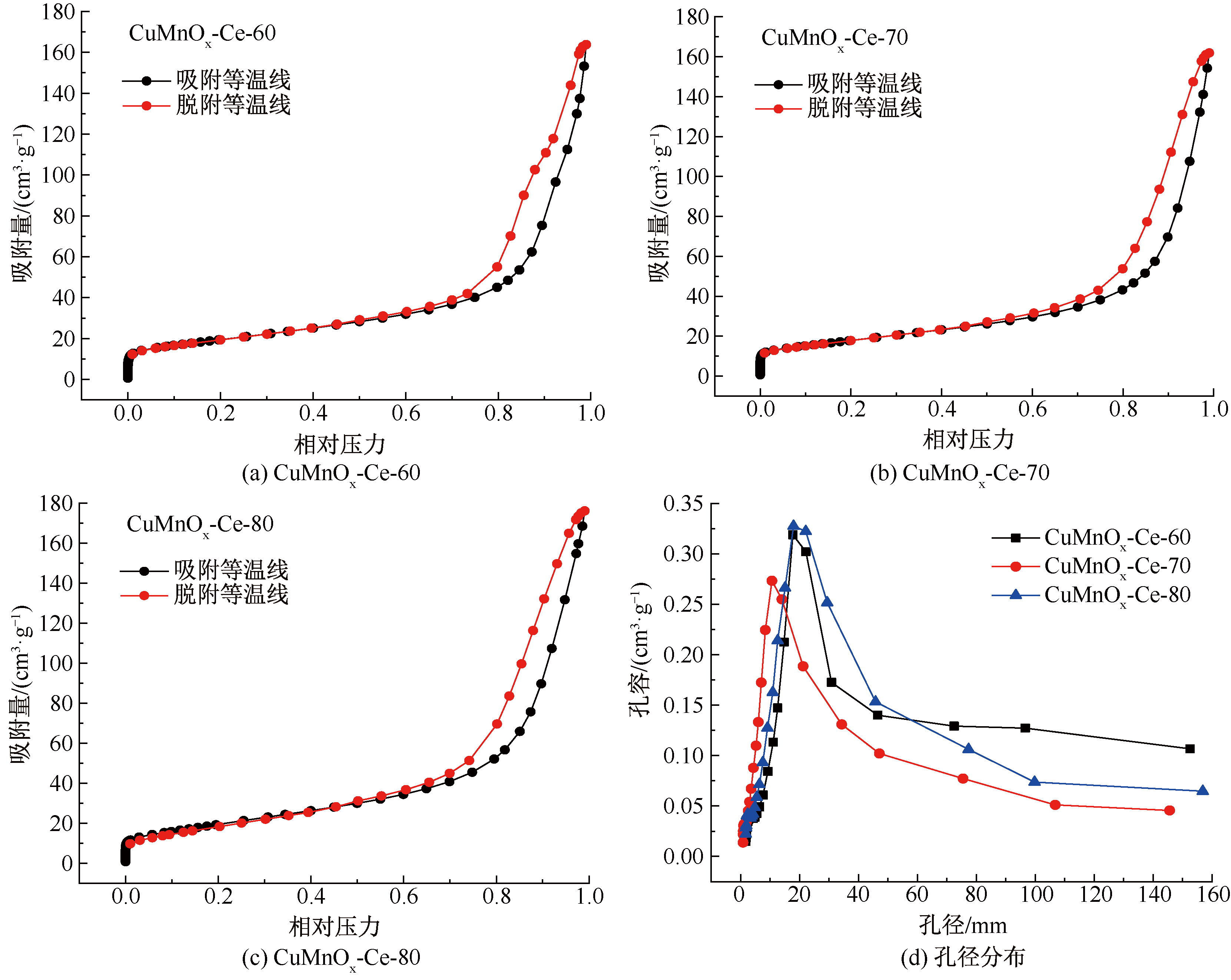
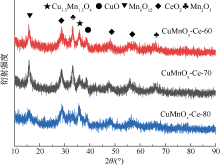
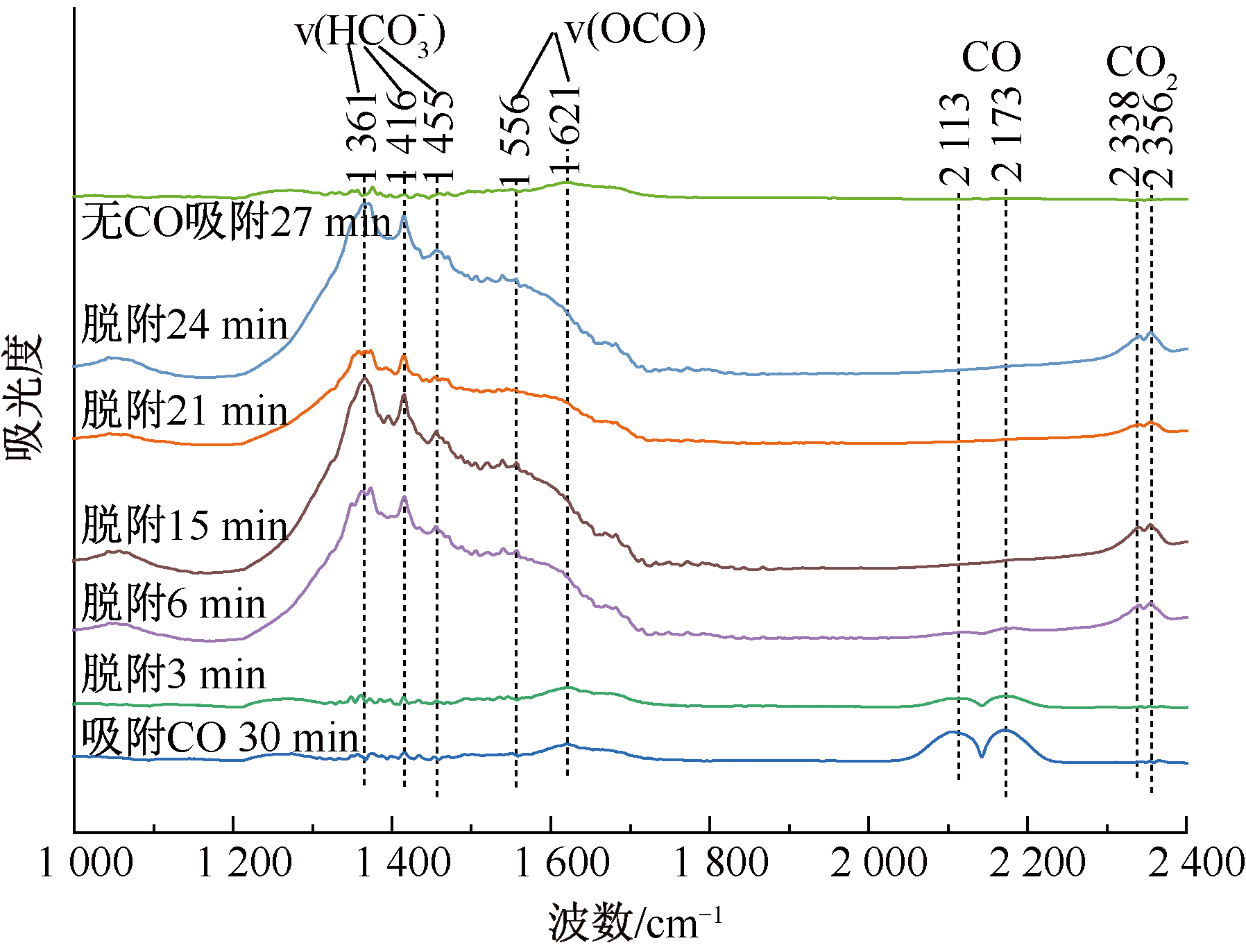
| [1] |
孙亚胜男. 煤矿井下空间CO消融特性及影响机制研究[D]. 阜新: 辽宁工程技术大学, 2021.
|
|
SUN Yashengnan. Research on CO elimination characteristics and influence mechanism in underground coal mine[D]. Fuxin: Liaoning Technical University, 2021.
|
| [2] |
CIMINO S, LISI L, TOTARELLA G, et al. Highly stable core-shell Pt-CeO2 nanoparticles electrochemically deposited onto fecralloy foam reactors for the catalytic oxidation of CO[J]. Journal of Industrial and Engineering Chemistry, 2018,66:404-410.
|
| [3] |
胡嘉. Pd/Cu修饰OMS-2催化剂低温催化氧化CO性能研究[D]. 昆明: 昆明理工大学, 2019.
|
|
HU Jia. Study on low temperature catalytic oxidation of CO over OMS-2 catalyst modified by Pd/Cu[D]. Kunming: Kunming University of Science and Technology, 2019.
|
| [4] |
黄志超, 王际童, 马成, 等. 低负载Pd/Al2O3催化剂的制备及其对CO室温催化性能研究[J]. 工业催化, 2021, 29(2):33-41.
doi: 10.3969/j.issn.1008-1143.2021.02.006
|
|
HUANG Zhichao, WANG Jitong, MA Cheng, et al. Preparation of Pd/Al2O3 catalyst with low Pd loading amount for CO oxidation at room temperature[J]. Industrial Catalysis, 2021, 29(2):33-41.
doi: 10.3969/j.issn.1008-1143.2021.02.006
|
| [5] |
GULYAEV R V, SLAVINSKAYA E M, NOVOPASHIN S A, et al. Highly active PdCeO composite catalysts for low-temperature CO oxidation, prepared by plasma-arc synthesis[J]. Applied Catalysis B:Environmental, 2014,147:132-143.
|
| [6] |
柯国洲. Au-Ag/SBA-15催化剂的制备及其对CO催化氧化性能的研究[D]. 大连: 大连理工大学, 2013.
|
|
KE Guozhou. Preparation of Au-Ag/SBA-15 catalyst and investigation of its catalytic performance for CO oxidation[D]. Dalian: Dalian University of Technology, 2013.
|
| [7] |
YEN Chunwan, LIN Mengliang, WANG Aiqin, et al. CO oxidation catalyzed by Au-Ag bimetallic nanoparticles supported in mesoporous silica[J]. The Journal of Physical Chemistry C, 2009, 113(41):17 831-17 839.
|
| [8] |
MOBINI S, MESHKANI F, REZAEI M. Supported Mn catalysts and the role of different supports in the catalytic oxidation of carbon monoxide[J]. Chemical Engineering Science, 2019,197:37-51.
|
| [9] |
SUN Yashengnan, ZHOU Xihua, BAI Gang, et al. Removal of CO generated by a gas explosion using a Cu-Mn elimination agent[J]. ACS Omega, 2021, 6(24):16 140-16 150.
|
| [10] |
陈然, 高晓亚, 王晶, 等. Ce改性Fe2O3催化剂对CO催化氧化的影响[J]. 化工进展, 2017, 36(10):3737-3742.
doi: 10.16085/j.issn.1000-6613.2017-0090
|
|
CHEN Ran, GAO Xiaoya, WANG Jing, et al. Effect of Ce modified Fe2O3 catalyst on CO catalytic oxidation[J]. Chemical Progress, 2017, 36(10):3737-3742.
|
| [11] |
申宏鹏, 黄金花, 李磊, 等. CuO-CeO2/MOx-Al2O3催化剂催化CO氧化性能的研究[J]. 应用化工, 2020, 49(9):2265-2269.
|
|
SHEN Hongpeng, HUANG Jinhua, LI Lei, et al. Study on catalytic performance of CuO-CeO2/MOx-Al2O3 catalyst in carbon monoxide oxidation[J]. Applied Chemical Industry, 2020, 49(9):2265-2269.
|
| [12] |
刘小元. CeO2基催化剂的制备及其CO氧化性能研究[D]. 合肥: 中国科学技术大学, 2019.
|
|
LIU Xiaoyuan. Synthesize of CeO2-based catalysts and their CO oxidation[D]. Hefei: University of Science and Technology of China, 2019.
|
| [13] |
WANG Beibei, ZHANG Hui, XU Wei, et al. Nature of active sites on Cu-CeO2 catalysts activated by high-temperature thermal aging[J]. ACS Catalysis, American Chemical Society, 2020, 10(21):12 385-12 392.
|
| [14] |
DEY S, DHAL G C, MOHAN D, et al. Cobalt doped CuMnOx catalysts for the preferential oxidation of carbon monoxide[J]. Applied Surface Science, 2018,441:303-316.
|
| [15] |
ZHENG Yan'e, LI Kongzhai, WANG Hua, et al. Structure dependence and reaction mechanism of CO oxidation: a model study on macroporous CeO2 and CeO2-ZrO2 catalysts[J]. Journal of Catalysis, 2016,344:365-377.
|
| [16] |
WU Ke, FU Xinpu, YU Wenzhu, et al. Pt-Embedded CuOx-CeO2 multicore-shell composites: interfacial redox reaction-directed synthesis and composition-dependent performance for co oxidation[J]. ACS Applied Materials & Interfaces, 2018, 10(40):34 172-34 183.
|