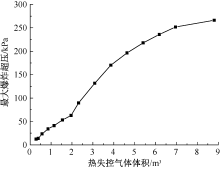
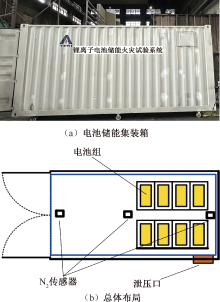
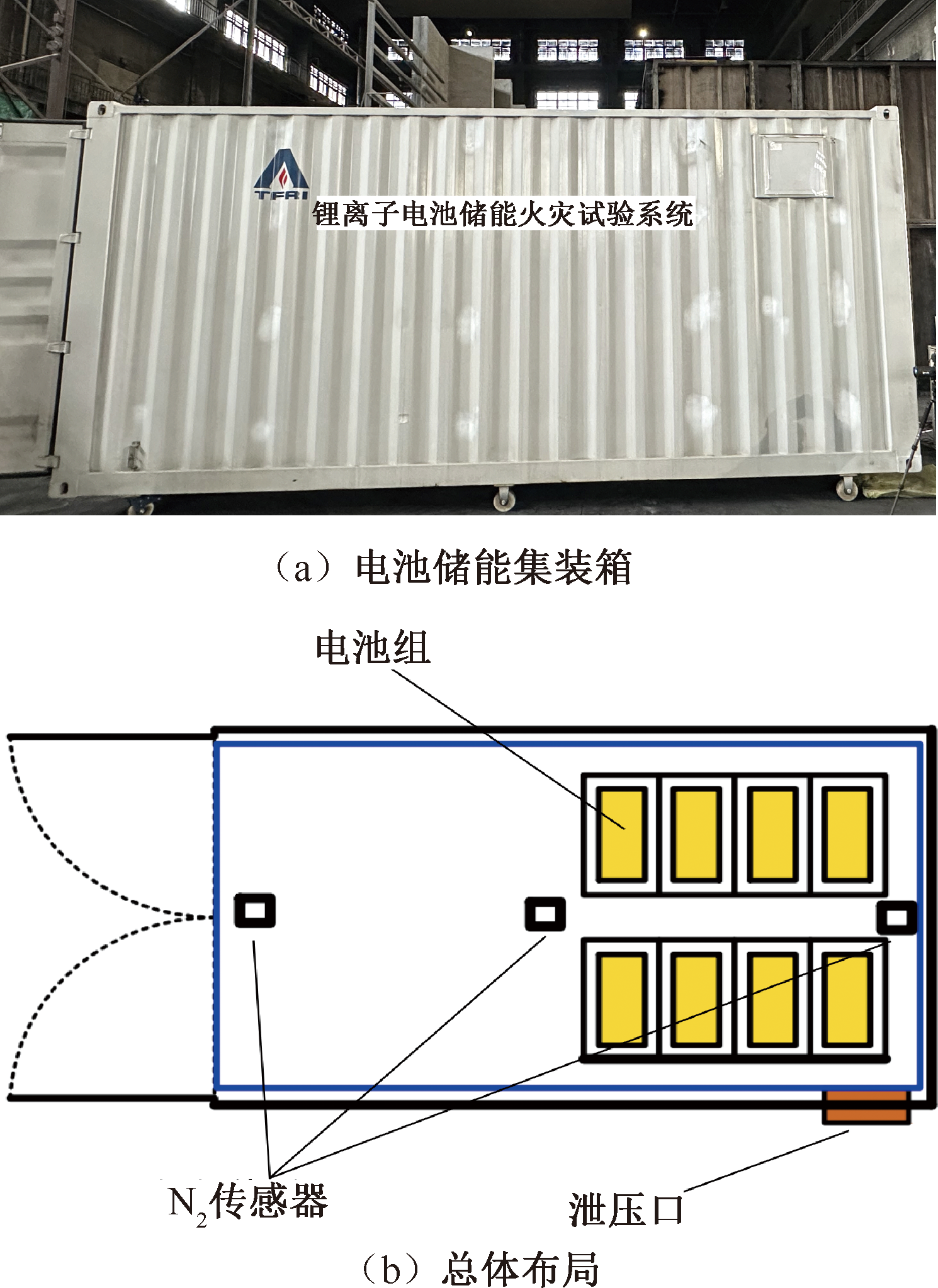
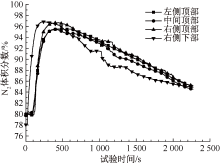
| [1] |
FENG Xuning, OUYANG Minggao, LIU Xiang, et al. Thermal runaway mechanism of lithium ion battery for electric vehicles: a review[J]. Energy Storage Materials, 2018, 10: 246-267.
doi: 10.1016/j.ensm.2017.05.013
|
| [2] |
LIANG Yeru, ZHAO Chenzi, YUAN Hong, et al. A review of rechargeable batteries for portable electronic devices[J]. InfoMat, 2019, 1: 6-32.
doi: 10.1002/inf2.v1.1
|
| [3] |
WANG Qingsong, MAO Binbin, STOLIAROV S I, et al. A review of lithium ion battery failure mechanisms and fire prevention strategies[J]. Progress in Energy and Combustion Science, 2019, 73: 95-131.
doi: 10.1016/j.pecs.2019.03.002
|
| [4] |
SUN Pieyi, BISSCHOP R, NIU Huichang, et al. A review of battery fires in electric vehicles[J]. Fire Technology, 2020, 56: 1361-1410.
doi: 10.1007/s10694-019-00944-3
|
| [5] |
BOEHMER H R, KLASSEN M S, OLENICK S M. Fire hazard analysis of modern vehicles in parking facilities[J]. Fire Technology, 2021, 57(5): 2097-2127.
doi: 10.1007/s10694-021-01113-1
|
| [6] |
SOMANDEPALLI V, MARR K, HORN Q. Quantification of combustion hazards of thermal runaway failures in lithium ion batteries[J]. SAE International Journal of Alternative Powertrains, 2014, 3(1): 98-104.
|
| [7] |
WANG Huaibin, XU Hui, ZHANG Zelin, et al. Fire and explosion characteristics of vent gas from lithium ion batteries after thermal runaway: a comparative study[J]. ETransportation, 2022, 13: DOI: 10.1016/j.etran.2022.100190.
|
| [8] |
WANG Qingsong, SHAO Guangzheng, DUAN Qiangling, et al. The efficiency of heptafluoropropane fire extinguishing agent on suppressing the lithium titanate battery fire[J]. Fire Technology, 2016, 52: 387-396.
doi: 10.1007/s10694-015-0531-9
|
| [9] |
XU Jiajia, GUO Pengyu, DUAN Qiangling, et al. Experimental study of the effectiveness of three kinds of extinguishing agents on suppressing lithium ion battery fires[J]. Applied Thermal Engineering, 2020, 171: DOI: 10.1016/j.applthermaleng.2020.115076.
|
| [10] |
庄卫强, 朱顺兵, 李勋. 惰性气体结合细水雾抑制锂离子电池燃烧试验研究[J]. 中国安全科学学报, 2019, 29(11):51-57.
doi: 10.16265/j.cnki.issn1003-3033.2019.11.009
|
|
ZHUANG Weiqiang, ZHU Shunbing, LI Xun. Experimental study on inhibition of lithium ion battery combustion by inert gas combined with water mist[J]. China Safety Science Journal, 2019, 29(11):51-57.
doi: 10.16265/j.cnki.issn1003-3033.2019.11.009
|
| [11] |
HUANG Zonghou, LIU Pengjie, DUAN Qiangling, et al. Experimental investigation on the cooling and suppression effects of liquid nitrogen on the thermal runaway of lithium ion battery[J]. Journal of Power Sources, 2021, 495: DOI: 10.1016/j.jpowsour.2021.229795.
|
| [12] |
SPOTNIITZ R, FRANKLIN J. Abuse behavior of high-power, lithium ion cells[J]. Journal of Power Sources, 2003, 113(1): 81-100.
doi: 10.1016/S0378-7753(02)00488-3
|
| [13] |
KIM G H, PESARAN A, SPOTNITZ R. A three-dimensional thermal abuse model for lithium ion cells[J]. Journal of Power Sources, 2007, 170(2): 476-489.
doi: 10.1016/j.jpowsour.2007.04.018
|
| [14] |
程志翔, 曹伟, 户波, 等. 储能用大容量磷酸铁锂电池热失控行为及燃爆传播特性[J]. 储能科学与技术, 2023, 12 (3): 923-933.
doi: 10.19799/j.cnki.2095-4239.2022.0690
|
|
CHENG Zhixiang, CAO Wei, HU Bo, et al. Thermal runaway and explosion propagation characteristics of large lithium iron phosphate battery for energy storage station[J]. Energy Storage Science and Technology, 2023, 12 (3): 923-933.
doi: 10.19799/j.cnki.2095-4239.2022.0690
|
| [15] |
LARSSON F, BERTILSSON S, FURLANI M, et al. Gas explosions and thermal runaways during external heating abuse of commercial lithium ion graphite-LiCoO2 cells at different levels of ageing[J]. Journal of Power Sources, 2018, 373: 220-231.
doi: 10.1016/j.jpowsour.2017.10.085
|
| [16] |
ZOU Kaiyu, LU Shouxiang, CHEN Xiao, et al. Thermal and gas characteristics of large-format LiNi 0.8Co 0.1Mn 0.1O 2 pouch power cell during thermal runaway[J]. Journal of Energy Storage, 2021, 39: DOI: 10.1016/j.est.2021.102609.
|
| [17] |
MISHRA D, SHAH K, JAIN A. Investigation of the impact of flow of vented gas on propagation of thermal runaway in a Li-ion battery pack[J]. Journal of Electrochemical Society, 2021, 168: DOI: 10.1149/1945-7111/2021/168(6)/060555.
|
| [18] |
YUAN Liming, DUBANIEWICZ T, ZLOCHOWER I, et al. Experimental study on thermal runaway and vented gases of lithium ion cells[J]. Process Safety and Environmental Protection, 2020, 144: 186-192.
doi: 10.1016/j.psep.2020.07.028
|
| [19] |
AQ/T 3046—2013,化工企业定量风险评价导则[S].
|
|
AQ/T 3046-2013, Guidelines for quantitative risk assessment of chemical enterprises[S].
|